Rule Breakers - s3.amazonaws.com
advertisement

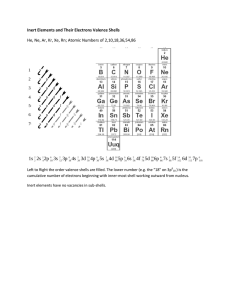
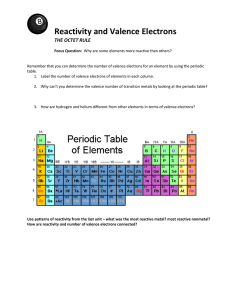
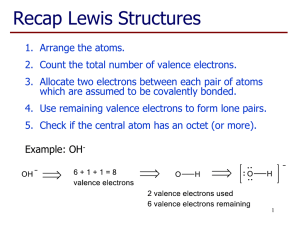
Rebel Molecules Some molecules disobey octet rule C, N, O, and F are mostly rule keepers 1) Odd Number of Valence Electrons 2) Incomplete Octets 3) Expanded Valence Shells Not every electron is in a pair All atoms in the molecule cannot have their octet satisfied Ex. NO, NO2 Molecules with odd valence electron #, not stable Very reactive Intermediate compounds in a chemical reaction, short-living Not enough electrons to go around ! Not every atom can achieve the octet rule as a result Reactive Commonly Be, B, and Al GENERALLY, A) All Lewis Structures are not necessarily equal for resonance hybrid B) Some resonance structures contribute more to the structure of the resonance hybrid than others. **applies only to elements in 3+ periods of periodic table ** Valence shell incorporates “d subshell region” (up to 18 electrons) More than 8 electrons shared Add valence shells if more than 8 valence electrons are around the central atom ** Applied when not possible to draw Lewis structure without expanding the shells ** Complete the Lewis Structure for BrF5 There is an error in each of these structures. What is it? Only one Lewis Structure is correct. Identify it and state the problem (s) with the other structures. Read pp. 361-366 Problems pp. 381-382 #53-54, 59-60 For Thursday, read over lab procedure