Periodic Trends
advertisement

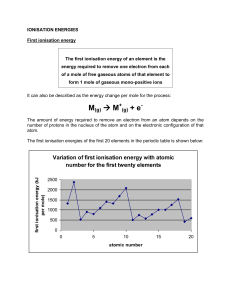
LG: I can describe trends in the periodic table and explain the reasons for these patterns Difficult to measure because outer edge of atom is not strictly defined Determined by measuring the distance between two nucleii and dividing by two Trends Increases from right left Increases from top bottom Every electron experiences a force of attraction toward the nucleus, called the effective nuclear charge. As you move down a group, we add energy levels that are progressively further from the nucleus Lower energy levels ‘shield’ outer electrons from the pull of the nucleus Elements gain or lose electrons to form ions The ionic radius of an element is always different than the atomic radius. In general: Metals lose electrons to form smaller cations Nonmetals gain electrons to form larger anions When an element loses electrons to obtain a full valence shell the resulting ion is smaller because: There are fewer electrons so each one experiences a greater effective nuclear charge There is one less electron shell, so all electrons are closer to nucleus When an element gains e-, there is a lower effective nuclear charge I.E. is the energy required to remove a valence e E.N. measures the tendency of an atom to gain e I.E. and E.N. both increase from left right and from bottom top I.E. and E.N. decrease from top bottom because valence e- are further from the nucleus Electrons are easier to remove More difficult to attract e- from other atoms I.E. and E.N. increase from left right because there is a larger effective nuclear charge Stronger hold on e Stronger pull on e- from other atoms Li, Na, K What happens when alkali metals are put in water? Which one is the most reactive? Why? Video (Brainiac) http://www.youtube.com/watch?v=m55kgyAp YrY New Book: Pg. 41 #1 - 7, 10