Alkanes
advertisement
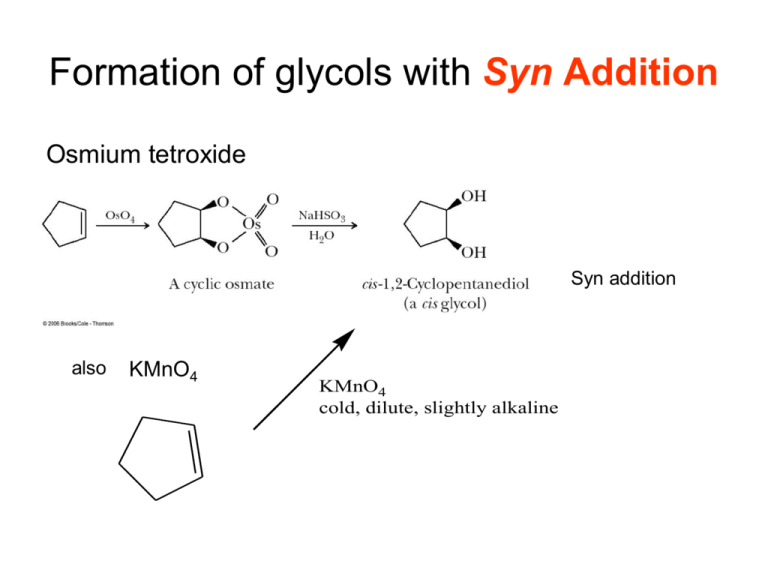
Formation of glycols with Syn Addition Osmium tetroxide Syn addition also KMnO4 KMnO4 cold, dilute, slightly alkaline Anti glycols Using a peracid, RCO3H, to form an epoxide which is opened by aq. acid. H+ PhCO3H, a peracid H2O O O H HO epoxide The protonated epoxide is analagous to the cyclic bromonium ion. O O OH Peracid: for example, perbenzoic acid OH An example PhCO3H O + O (S)-3-methylcyclohex-1-ene aq. acid chiral, optically active OH OH OH OH OH OH Diastereomers, separable (in theory) by distillation, each optically active OH OH Are these unique? Ozonolysis R3 R1 1. O3 R1 R3 O R4 R2 2. (CH3)2S R4 + O R2 Reaction can be used to break larger molecule down into smaller parts for easy identification. Ozonolysis Example For example, suppose an unknown compound had the formula C8H12 and upon ozonolysis yielded only 3-oxobutanal. What is the structure of the unknown? The hydrogen deficiency is 18-12 = 6. 6/2 = 3 pi bonds or rings. The original compound has 8 carbons and the ozonolysis product has only 4 Conclude: Unknown two 3-oxobutanal. Unknown ozonolysys C8H12 O O O O O Simply remove the new oxygens and join to make double bonds. But there is a second possibility. Another Example 2. An unknown compound (derived from the gall bladder of the gila monster) has the formula C10H14 . When subjected to ozonolysis the following compound is isolated O Suggest a reasonable structure for the unknown. d Hydrogen Deficiency = 8. Four pi bonds/rings. O a O c Unknown has no oxygens. Ozonolysis product has four. Each double bond produces two carbonyl groups. Expect unknown to have 2 pi bonds and two rings. b O To construct unknown cross out the oxygens and then connect. But there are many ways the connections can be made. a-b & c-d d a-d & b-c a-c & b-d c a c Look for a structure that obeys the isoprene rule. d d a c b a b b Mechanism Consider the resonance structures of ozone. Electrophile capability. Nucleophile capability. O O O O O O O O O O O O These two, charged at each end, are the useful ones to think about. Mechanism - 2 O O O O O O O O O O O O Mechanism - 3 Mechanism - 4 Hydrogenation No regioselectivity Syn addition Heats of Hydrogenation Consider the cis vs trans heats of hydrogenation in more detail… Heats of Hydrogenation - 2 The trans alkene has a lower heat of hydrogenation. Conclusion: Trans alkenes with lower heats of hydrogenation are more stable than cis. We saw same kind of reasoning when we talked about heats of combustion of isomeric alkanes to give CO2 and H2O By same reasoning higher degree of substitution provide lower heat of hydrogenation and are, therefore, more stable. Reduced heat of Hydrogenation Increasing substitution Heats of Hydrogenation Acid Catalyzed Polymerization Principle: Reactive pi electrons (Lewis base) can react with Lewis acid. Recall H + H Which now reacts with a Lewis base, such as halide ion to complete addition of HX yielding 2-halopropane Variation: there are other Lewis bases available. THE ALKENE. + the carbocation is an acid! The new carbocation now reacts with a Lewis base such as halide ion to yield halide ion to yield 2-halo-4-methyl pentane (dimerization) but could react with another propene to yield higher polymers. Examples of Synthetic Planning OH Give a synthesis of 2-hexanol from any alkene. Planning: Alkene is a hydrocarbon, thus we have to introduce the OH group How is OH group introduced (into an alkene): hydration What are hydration reactions and what are their characteristics: •Mercuration/Reduction: Markovnikov •Hydroboration/Oxidation: Anti-Markovnikov and syn addition What alkene to use? Must involve C2 in double bond. Which reaction to use with which alkene? Markovnikov rule can be applied here. CH vs CH2. Want Markovnikov! Use Mercuration/Reduction!!! Markovnkov Rule cannot be used here. Both are CH. Do not have control over regioselectivity. Do not use this alkene. For yourself : how would you make 1 hexanol, and 3-hexanol? Another synthetic example… How would you prepare meso 2,3 dibromobutane from an alkene? Analysis: Alkene must be 2-butene. But wait that could be either cis or trans! We want meso. Have to worry about stereochemistry Know bromine addition to an alkene is anti addition (cyclic bromonium ion) trans cis Br2 Br2 Br Br H H Br rotate lower unit + enantiomer Br H Br Br H Br H Br meso This worked! How about starting with the cis? racemic mixture This did not work, gave us the wrong stereochemistry! Addition Reaction General Rule… Characterize Reactant as cis or trans, C or T Characterize Reaction as syn or anti, S or A Characterize Product as meso or racemic mixture, M or R Relationship Characteristics can be changed in pairs and C A R will remain true. A C R Want meso instead?? Have to use trans. Two changed!! Br2 H Br + enantiomer Br H A T M cis Br2 racemic mixture Br H Br H trans meso Alkynes Structure sp hybridization Acidity of Terminal Alkynes Stronger base Weaker base Other strong bases that will ionize the terminal alkyne: Not KOH Important Synthetic Method: Dehydrohalogenation 1. Dehydrohalogenation… An alkyl halide can eliminate a hydrogen halide molecule, HX, to produce a pi bond. Recall that HX can be added to a double bond to make an alkyl halide. HX can also be removed by strong base, called dehydrohalogenation. Preparation of alkene Strong base RCH=CHR + HX RCHXCH2R Or rewriting RCHBrCH2R base RCH=CHR Also, if we start with a vinyl halide and a very strong base (vinyl halides are not very reactive). NaH RCH=CHBr RCCH Synthetic planning (Retrosynthesis) Work Backwards….. Trace the reactions sequence from the desired product back to ultimate reactants. Br Br2 H H H NaNH2 NaNH2 Br CH3 Starting reactant CH3 Br H prop-1-yne CH3 CH3 Target molecule. Overall Sequence converts alkene alkyne H But typical of synthetic problems side reaction occurs to some extent and must be taken into account. C H H H More Sythesis: Nucleophilic Substitution Use the acidity of a terminal alkyne to create a nucleophile which then initiates a substitution reaction. Note that we still have an acidic hydrogen and, thus, can react with another alkyl group in this way to make RCCR’ Alkyl halides can be obtained from alcohols Reactions: alkyne with halogen RCCR + Br2 RBrC=CBrR No regioselectivity with Br2. Stereoselective for trans addition. Reactions: Addition of HX The expected reaction sequence occurs, formation of the more stable carbocation. Markovnikov orientation for both additions. Now for the mechanism…. Mechanism The expected reaction sequence occurs, formation of the more stable carbocation. Addition of the second mole, another example of resonance. Reactions: Acid catalyzed Hydration (Markovnikov). Markovnikov addition, followed by tautomerism to yield, usually, a carbonyl compound. Reactions: Anti Markovnikov Hydration of Alkynes, Regioselectivity BH3 overall: R R' Step 1 R H R' B H2O2, NaOH Step 2 RCH2CR' o Similar to formation of an anti-Markovnikov alcohol from an alkene Step 1, Internal Alkyne: addition to the alkyne with little or no regioselectivity issue. Alternatively Asymmetric, terminal, alkyne if you want to have strong regioselectivity then use a borane with stronger selectivity for more open site of attack. Less exposed site. More exposed site. sia2BH Aldehyde not ketone. Tautomerism, enol carbonyl Step 2, Reaction of the alkenyl borane with H2O2, NaOH would yield an enol. Enols are unstable and rearrange (tautomerize) to yield either an aldehyde or ketone. catalyzed by base or acid H2O2 H H H H NaOH OH O B enol either an aldehyde or a ketone Overall… internal alkyne ketone (possibly a mixture, next slide) Terminal alkyne aldehyde Examples Used to insure regioselectivity. As before, for a terminal alkyne. But for a non-terminal alkyne frequently will get two different ketones Get mixture of alkenyl boranes due to low regioselectivity. Reduction, Alkyne Alkene 1. Catalytic Hydrogenation If you use catalysts which are also effective for alkene hydrogenation you will get alkane. You can use a reduced activity catalyst (Lindlar), Pd and Pb, which stops at the alkene. You obtain a cis alkene. Syn addition Reduction - 2 2. Treatment of alkenyl borane with a carboxylic acid to yield cis alkene. BH3 CH3CO2H hex-3-yne H B Instead of H2O2 / NaOH Alkenyl borane 3. Reduction by sodium or lithium in liquid ammonia to yield the trans alkene. Plan a Synthetic Sequence Retrosynthesis Synthesize butan-1-ol from ethyne. Work backward from A big the alkyne target can molecule. be formed via nucleophilic Is read as “comes from”. substitution. This is the chance to make thedone. C-C Major problem: make big from small. Be alert for Catalytic when the “disconnect” can be 1. BH3 bond we need. Lindlar OH 2. H2O2, reduction NaOH YES! butan-1-ol Target molecule Convert ethyne to anion Do a “disconnect” here. and react with EtBr. Catalytic Br reduction Addition Lindlar of HBr. bromoethane Now, fill in the “forward reaction” details Can we get alkyne from smaller Not How yet! about Soan joining how can molecules we get it? to get an alkene? Ask yourself! Do we know how tomolecules? join any two Not yet!! So howtogether can we to getyield an alkene? molecules an alcohol? ethyne