Alkene/Alkyne Reactions: Mechanisms, Naming, and Practice
advertisement

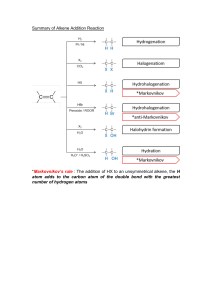
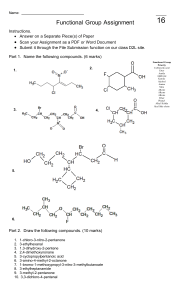
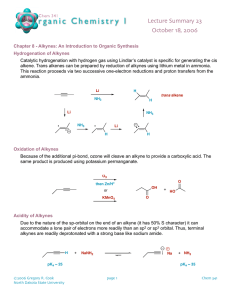
Alkene/Alkynes reactions - Nomenclature - Reaction mechanisms (Hydration, hydrohalogenation, halogenation) for alkenes and alkynes. Halo-hydrin reaction of alkenes and Alkylation of alkynes. - Predict reagents, predict reactants, predict products. - Focus on functional groups. - Physical properties, acidity of alkynes. Alkene/Alkyne naming • Find the longest chain involved C=C and alkyne. • Give priority to smaller numbering of C=C or alkyne • Find the substituents and arrange by alphabetical order. • Combine substituents. • Assign R/S and E/Z if there’s any. Alkene/Alkyne drawing 4-cyclobutyl-cyclooctyne • Draw parent structure. • Draw properly in which carbon attached to triple bond be linear. • Draw substituent. • Add substituent by numbering across triple bond. Alkene/Alkyne drawing 4-cyclobutyl-cyclooctyne • Draw parent structure. • Draw properly in which carbon attached to triple bond be linear. • Draw substituent. • Add substituent by numbering across triple bond. Alkene/Alkyne drawing 1-(2-methylcyclopentyl)-1-octyne • Draw parent structure. • Draw substituent. Halogenation and Halohydrin reactions Bromination and Halohydrin reactions mechanism Halo-hydrin reaction Alkene reactions (functional group changes) Hydrohalogenation and Hydration H2O Alkoxylation Carbocation rearrangement • Vinyl group or C=C attach to a tertiary C or quarternary C. • H (tertiary C) or CH3/Alkyl (quarternary C) shift to form more stable carbocation. • Applicable to hydration, hydro-halogenation, alkoxylation. Carbocation rearrangement Carbocation/Ring expansion • Vinyl group or C=C attach to a quarternary C. • Bond shift to form more stable carbocation. Hydroboration, BH3/THF or B2H6, R2BH • OH preferred less substituted site. • No rearrangement. • H and OH always cis to each others, syn addition, resulting trans-product (CH3 and OH trans to each others) in this case. Epoxidation, hydrogenation • Same side for epoxide, syn addition. • H is cis to each other for hydrogenation, syn addition. Ozonolysis • Cut double bond off. • Add O to each side. • Mono-substituted side gives aldehyde. Di-substituted side gives ketone Alkene reactions practice Alkene reactions practice Halo-hydrin practice What’s the major product/s? What’s the major product/s? Alkyne reactions Alkene and Alkynes additions Bromination/Halogenation of alkene/alkynes mechanism Hydro-halogenation, HCl, HBr of alkene/alkyne mechanism Hydration H2O mechanism Hydrogenation of alkynes Don’t confuse by both of these Alkylation of terminal alkynes Don’t confuse by both of these Alkylation of terminal alkynes 1. 2 steps reaction. 2. Step one involves deprotonation of acidic terminal alkyne proton. 3. Step 2 involves nucleophilic substitution reaction in which the carbanion attacks the electrophile alkylbromide. 4. The electrophile has to be primary halides. Alkenes vs Alkyne reactions Reagents Alkene Alkynes H3O+/H2O Alcohol, more substituted Ketone, more substituted 1.BH3, 2.NaOH/H2O2 Alcohol, less substituted Aldehyde, less substituted HCl or HBr Cl or Br on more substituted Cl or Br on more substituted. If 2eq or more, 2x Cl or Br on more substituted. Br2 or Cl2 trans-dibromide or dichloride trans-dibromide or dichloride. If 2eq or more, 4 Cl or Br across 2 carbons. Stereo and Regioselectivity Reagents Stereoselective H3O+/H2O 1.BH3, 2.NaOH/H2O2 Regioselective Markovnikov Syn-addition HCl or HBr Anti-Markovnikov Markovnikov Br2 or Cl2 Anti-addition Br2 and H2O Anti-addition Markovnikov Cl2 and H2O Anti-addition Markovnikov mCPBA Syn-addition H2/Pd or Ni or Pt Syn-addition Example of questions Example of questions Alkylation of terminal alkynes, which combo? Multiple choice questions 1. Most stable carbocation? Multiple choice questions 1. Most stable carbocation? Multiple choice questions 1. Rank increasing acidity (from least acidic to most acidic)? Multiple choice questions 1. Which one follows anti-Markovnikov? 2. Which one will give H-shift or rearranged product? Multiple choice questions 1. Which will give syn product? 2. Which will give 2 diastereomers? Multiple choice questions 1. Which will NOT give 2 products? 2. Which will NOT have ketone product? GOOD LUCK! Have a great holiday!