Chemistry 201 - C
Alkynes: An Introduction to
Organic Synthesis
This presentation was created by
Professor Carl H. Snyder
Chemistry Department
University of Miami
Coral Gables, FL 33124
CSnyder@miami.edu
Copyright 2004 by Carl H. Snyder,
University of Miami. All rights
reserved.
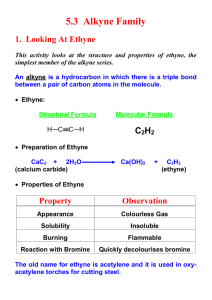
sp Hybridization
Structure of Acetylene
2 unhybridized p orbitals
2 sp orbitals
sp hybridized carbons
a linear molecule
Naming Alkynes
Alkyl, Alkenyl, and Alkynyl
Groups
1
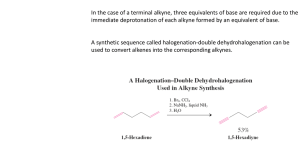
Creation of Triple Bond
Terminal And Internal Alkynes
Elimination of two HX from vicinal dihalide
Terminal alkyne -- Contains a triple bond
between C-1 and C-2: R-C≡CH
A hydrogen is bonded to an sp carbon.
Internal alkyne -- Both sp carbons are
bonded to carbon atoms: R-C≡C-R’
Additions To Alkynes - Addition of
HX
Addition of HX
Markovnikov orientation
Can occur either once or twice
acetic acid
Stereochemistry of Addition
Addition of HX to an internal
alkyne produces a trans product.
A vinylic carbocation is an intermediate.
Addition of X2
Addition of X2 can occur either once or twice
Produces a trans product
2
Addition of H2O -- H+ and Hg
Catalyzed
Aldehydes and Ketones
Acid-catalyzed, as in acid-catalyzed
hydration of alkenes
Also requires Hg catalysis
Involves enol intermediate
Aldehydes and Ketones
Keto-Enol Tautomerism
Ketone - R2C=O
Enol - HO- resides on sp2 carbon of C=C
Tautomerism - Rapid interconversion of constitutional
isomers
Keto-enol tautomerism usually favors the keto form
Terminal vs. Internal
Internal alkyne gives a mixture of two ketones.
Terminal vs. Internal
Internal alkyne gives a mixture of two ketones.
Because of Markovnikov orientation, a terminal
alkyne gives predominantly a methyl ketone.
3
Addition of H2O
Hydroboration/Oxidation Terminal Alkynes
Terminal alkynes produce only aldehydes on
hydroboration/oxidation.
Addition of H2O
Hydroboration/Oxidation Ketones from Internal Alkynes
A Choice of Products from A
Choice of Reagents
For a ketone from a terminal alkyne, use H+,
HgSO4 , H2O
For an aldehyde from a terminal alkyne, use
hydroboration/oxidation.
Addition of H2
Catalytic Reduction
Hydroboration/oxidation of an unsymmetrical alkyne,
R’-C≡C-R”, gives a mixture of two different ketones.
Addition of H2
Lithium And Ammonia
This method gives a trans alkene through anti
addition of H2
For a cis alkene, through syn addition, use the
Lindlar catalyst.
For an alkane, use H2 and Pd/C.
Acidity of Terminal
Alkynes
The C≡C-H of terminal alkynes is more acidic than any
other hydrogen of alkanes, alkenes or alkynes.
4
Stability of the Acetylide Anion
The greater the s character of the orbitial, the closer to
the nucleus it lies and the lower its energy.
Alkylation of The Acetylide
Anion
The reaction of an acetylide anion and a methyl
or a 1o aklyl halide results in the alkylation of
the acetylide anion.
Here the anion is methylated.
Mechanism of The Alkylation
Alkylation: Generality
Alkylation: Limitation
Organic Synthesis: Challenge
5
Organic Synthesis: Solution
Organic Synthesis: Strategy
What reaction converts an alkyne to an alkane?
What alkyne would you start with to obtain octane?
Organic Synthesis: Solution
Organic Synthesis: Solution
How would you convert 1-pentyne to 4-octyne?
With these steps you have converted 1-pentyne
to octane.
Problem #1
Problem #1 - Solution
Convert a 5-carbon, terminal alkyne into a
6-carbon, cis 2-alkene.
What’s the final step?
6
Problem #1 - Solution
Problem #1 - Solution
Problem #2
Problem #2 - Solution
Convert a 2-carbon alkyne into a 5-carbon, 2o
alkyl bromide.
Problem #2 - Solution
Problem #2 - Solution
7
Problem #2 - Solution
Problem #3
Convert a 2-carbon alkyne into a terminal,
6-carbon, unbranched alcohol
Problem #3 - Solution
Problem #3 - Solution
Problem #3 - Solution
End
Alkynes
8