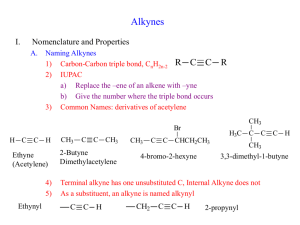
Alkynes: Structure, Nomenclature, and Reactions
advertisement
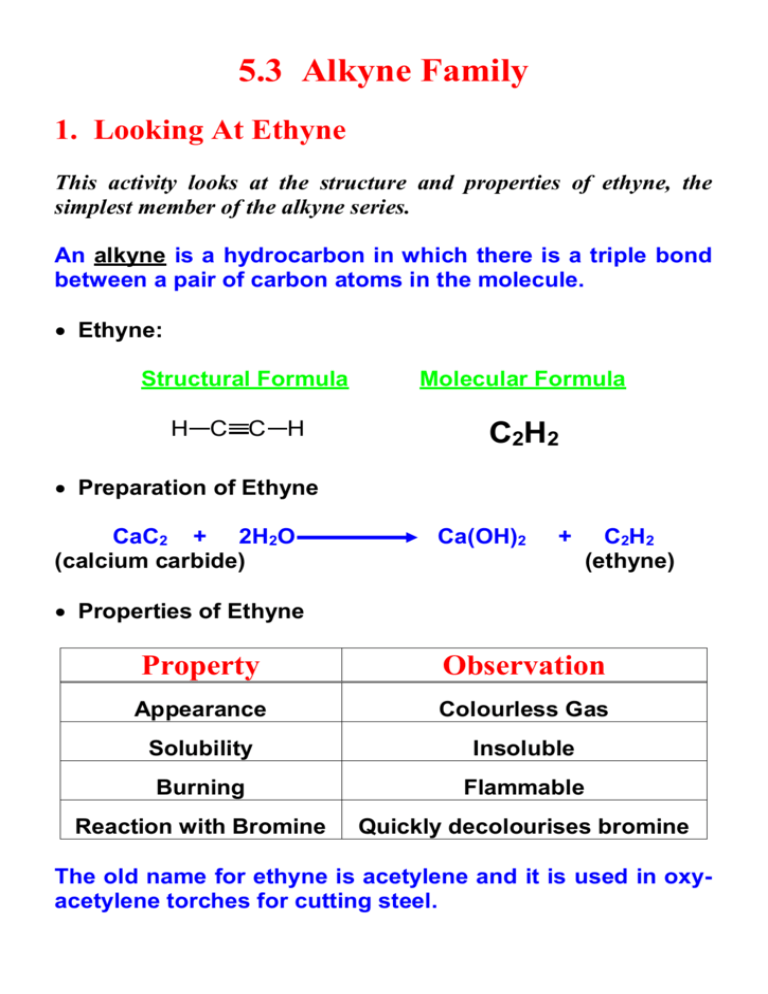
5.3 Alkyne Family 1. Looking At Ethyne This activity looks at the structure and properties of ethyne, the simplest member of the alkyne series. An alkyne is a hydrocarbon in which there is a triple bond between a pair of carbon atoms in the molecule. Ethyne: Structural Formula H C C H Molecular Formula C2H2 Preparation of Ethyne CaC2 + 2H2O (calcium carbide) Ca(OH)2 + C2H2 (ethyne) Properties of Ethyne Property Observation Appearance Colourless Gas Solubility Insoluble Burning Flammable Reaction with Bromine Quickly decolourises bromine The old name for ethyne is acetylene and it is used in oxyacetylene torches for cutting steel. 2. Structures and Names This activity is about the structures and systematic names of alkyne molecules. The general formula for the alkyne family is: CnH2n-2 But-1-yne: Structural Formula Molecular Formula H H H C C C C H H C4H6 H In branched alkynes, the main chain must contain the triple bond and the chain numbered to give the lowest position for the triple bond. *Branched Alkyne Structures* 3. Addition Reactions This activity looks at some addition reactions of alkynes and how these take place in two stages. Reaction of but-2-yne with chlorine. Stage One: H H H C C C C H H H Cl Cl H + Cl2 H H C C C C H H but-2-yne H 2,3-dichlorobut-2-ene If excess chlorine is available the reaction will continue to stage 2. Otherwise the reaction will stop here. The product of stage one is still unsaturated and so can add more chlorine to the double bond (stage two). Stage Two: H Cl Cl H H C C C C H H H Cl Cl H + Cl2 H 2,3-dichlorobut-2-ene H C C C C H H Cl Cl H 2,2,3,3-tetrachlorobutane Reaction of propyne with hydrogen chloride: Stage One: H Cl H H H C C C H + HCl C C C H H H propyne H 2-chloropropene Stage Two H Cl H H Cl H C C C H H C C C H H + HCl H 2-chloropropene H Cl H 2,2-dichloropropane Hydrogenation of 3-propylhex-1-yne: Stage One: CH2CH2CH 3 H + H2 H C C CH CH2CH2CH 3 3-propylhex-1-yne H H CH 2CH2CH3 C C CH CH2CH 2CH3 3-propylhex-1-ene Stage Two: H H H CH 2CH2CH3 + H2 C C CH CH2CH 2CH3 3-propylhex-1-ene H H CH2CH2CH3 H C C C CH 2CH2CH3 H H H 4-ethylheptane