Newspaper article, p
advertisement
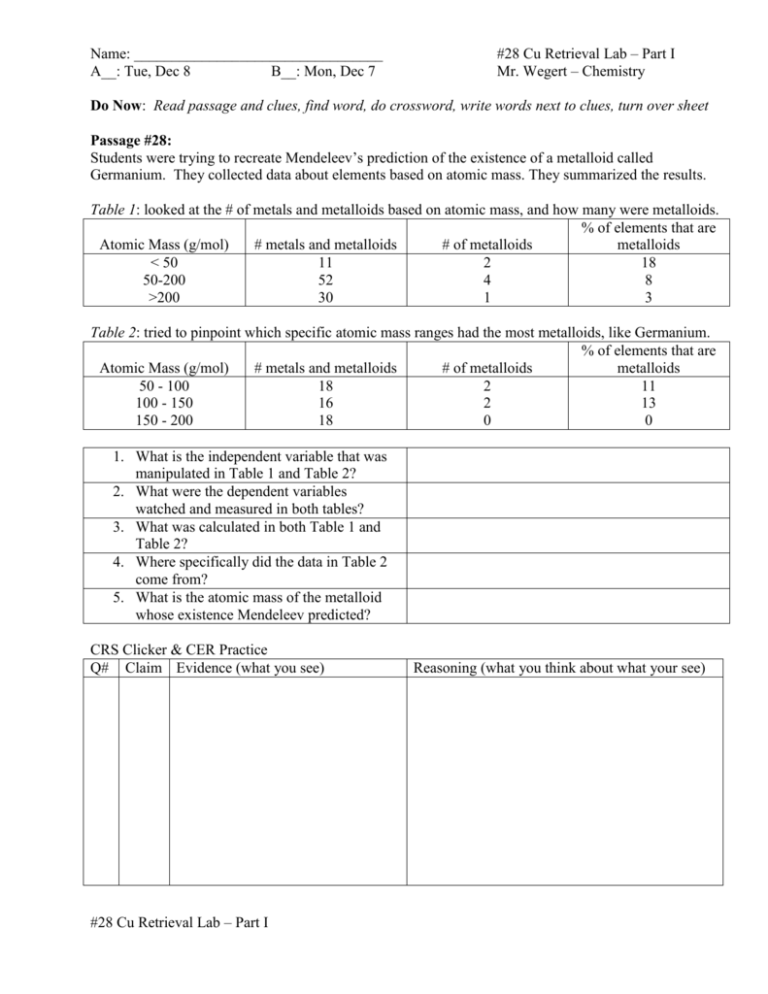
Name: _________________________________ A__: Tue, Dec 8 B__: Mon, Dec 7 #28 Cu Retrieval Lab – Part I Mr. Wegert – Chemistry Do Now: Read passage and clues, find word, do crossword, write words next to clues, turn over sheet Passage #28: Students were trying to recreate Mendeleev’s prediction of the existence of a metalloid called Germanium. They collected data about elements based on atomic mass. They summarized the results. Table 1: looked at the # of metals and metalloids based on atomic mass, and how many were metalloids. % of elements that are Atomic Mass (g/mol) # metals and metalloids # of metalloids metalloids < 50 11 2 18 50-200 52 4 8 >200 30 1 3 Table 2: tried to pinpoint which specific atomic mass ranges had the most metalloids, like Germanium. % of elements that are Atomic Mass (g/mol) # metals and metalloids # of metalloids metalloids 50 - 100 18 2 11 100 - 150 16 2 13 150 - 200 18 0 0 1. What is the independent variable that was manipulated in Table 1 and Table 2? 2. What were the dependent variables watched and measured in both tables? 3. What was calculated in both Table 1 and Table 2? 4. Where specifically did the data in Table 2 come from? 5. What is the atomic mass of the metalloid whose existence Mendeleev predicted? CRS Clicker & CER Practice Q# Claim Evidence (what you see) #28 Cu Retrieval Lab – Part I Reasoning (what you think about what your see) Purpose:_________________________________________________________________________ _________________________________________________________________________________ Procedure Checklist (in pairs): Data Table: Copper Retrieval Lab – Part I (mass in grams, g) 1. Mass of CuO + cup = 2. Mass of empty cup = 3. Calculate Mass of CuO = 4. Mass of large filter paper = 5. Mass of small filter paper = Homework Scratch Area: Labs Copper Lab Unbalanced Reaction Cu + O2 CuO Cu Retrieval Lab – Part I CuO + HCl → CuCl2 + H2O Cu Retrieval Lab – Part II CuCl2 + Zn → Cu + ZnCl Homework: Complete worksheet table above #28 Cu Retrieval Lab – Part I Balanced Reaction ____/10 pts










