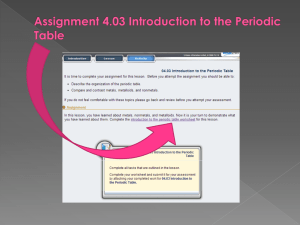
Elements and Their Properties
advertisement

chapter 17 Elements and Their Properties 3 section ● Mixed Groups What You’ll Learn the differences between metals, nonmetals, and metalloids ■ what allotropes are ■ the different crystal structures of carbon ■ why synthetic elements are important ■ Before You Read Have you ever seen a mixed-breed dog? What characteristics of different breeds did the dog have? Read to Learn read the section, highlight the name of each element group that is discussed. Then highlight the names and uses of some of the elements in that group. C Build Vocabulary ● Make a vocabulary Foldable like the one below to help you understand how the elements in the mixed groups are similar and different. Metalloids Boron Group Carbon Group Nitrogen Group Oxygen Group Synthetic Elements 306 Properties of Metalloids Can an element be a metal and a nonmetal? In some ways, elements called metalloids are. Metalloids share unusual characteristics. Metalloids are elements that can form ionic and covalent bonds with other elements and can have the properties of metals and nonmetals. For example, some metalloids can conduct electricity better than most nonmetals. However, they do not conduct electricity as well as some metals. This property gives them the name semiconductors. Except for aluminum, the metalloids are the elements on the periodic table that are located along the stair-step line. Metalloids can be found in Groups 13, 14, 15, 16, and 17. The Boron Group The metalloid boron is the first element in Group 13. You might find two boron compounds in your house. The first is borax. Borax is added to laundry detergents to soften water. The other is boric acid, an antiseptic. Compounds called boranes are used for jet and rocket fuel. CHAPTER 17 Elements and Their Properties 5 B 13 Al 31 Ga 49 ln 81 Tl Copyright © Glencoe/McGraw-Hill, a division of The McGraw-Hill Companies, Inc. Identify Elements As you Aluminum is a metal in Group 13. It is the most common metal in Earth’s crust. You have seen aluminum in soft drink cans, foil wrap, and cooking pans. Aluminum is strong and light and is used in making airplanes. The Carbon Group Atoms of the elements in Group 14 have four electrons in their outer energy levels. Other than this, the elements in the carbon group are very different. Carbon is a nonmetal, silicon and germanium are metalloids, and tin and lead are metals. 6 C 14 Si Picture This 1. Identify Circle the metalloids in the figure. 32 Ge 50 Sn 82 Pb Copyright © Glencoe/McGraw-Hill, a division of The McGraw-Hill Companies, Inc. Carbon Carbon occurs as an element in coal. Compounds of carbon make up oil and natural gas. Carbon can combine with oxygen to produce carbon dioxide, CO2. Plants use CO2 and sunlight to make food. All organic compounds contain carbon, but not all carbon compounds are organic. Silicon and Germanium The metalloid silicon is the second most common element in Earth’s crust. Most silicon is found in sand (SiO2). It also is found in almost all rocks and soil. Allotropes are different forms of the same element that have different molecular structures. Molecular structure is how the atoms in a molecule are arranged. Silicon has two allotropes. One allotrope of silicon is a hard, gray substance. The other is a brown powder. Because it is a semiconductor, silicon is used in many electronic devices, such as transistors and computer chips. A semiconductor is an element that conducts electric current under certain conditions. Germanium is the other metalloid in the carbon group. It is used with silicon to make semiconductors. Tin and Lead Tin is used to coat other metals to prevent corrosion. It also is mixed with other metals to make bronze and pewter. Tin cans are made of steel coated with tin. Lead is a soft metal that was once used to make paints. Lead is no longer used in paint because it is toxic. 2. Explain What are semiconductors? What are the allotropes of carbon? What do a diamond ring and a pencil have in common? The diamond in a ring and the graphite in a pencil are carbon. There are three known allotropes of carbon. They are diamond, graphite, and buckminsterfullerene (BUK mihn stur ful ur een). Reading Essentials 307 3. Apply Why do you think some saw blades are coated in diamond powder? Diamonds Diamonds are clear and extremely hard. In fact, they are the hardest things in the world. In a diamond, each carbon atom is bonded to four other carbon atoms. The bonded carbon atoms form a geometric shape called a tetrahedron. Many of these tetrahedrons join together to form a giant molecule. In this molecule, the atoms are held tightly together in a strong crystalline structure. This strucure is why diamonds are so hard. Graphite Graphite is a black powder that is made of hexagonal layers of carbon atoms. In the hexagons, each carbon atom is bonded to three other carbon atoms. The fourth electron in each atom is bonded weakly to the layer above or below it. These weak bonds let the layers of carbon atoms slide easily past each other. This makes graphite very slippery. Buckminsterfullerene Buckminsterfullerene was discovered in the 1980s. It forms molecules that are shaped like soccer balls. It is named after R. Buckminster Fuller, who designed structures with similar shapes. Scientists have used buckministerfullerene to make tiny tubes called nanotubes. They are one-billionth of a meter in diameter. You can stack tens of thousands of nanotubes to get the thickness of one sheet of paper. Nanotubes might be used some day to make smaller, faster computers. 4. Use Percentages Assume you inhale in about 6 L of air per minute. How much nitrogen do you breathe in one minute? 308 The Nitrogen Group Group 15 is known as the 7 nitrogen family. Atoms of N each element in the group 15 have five electrons in their P outer energy levels. Group 33 15 elements usually share As their electrons in covalent 51 bonds with other elements. Sb About 80 percent of the 83 air you breathe is nitrogen. Bi In the air, nitrogen exists as diatomic molecules, N2. All organisms need nitrogen compounds to live. Even though you breathe it, your body cannot use nitrogen in its diatomic form. It must be in the form of a nitrogen compound. Nitrogen often is used to make compounds called nitrates and ammonia, NH4. Nitrates are compounds that contain the nitrate ion, NO3. Nitrates and ammonia are important ingredients of fertilizers. CHAPTER 17 Elements and Their Properties Copyright © Glencoe/McGraw-Hill, a division of The McGraw-Hill Companies, Inc. Applying Math How are elements of the nitrogen group used? Phosphorus is a nonmetal that has three allotropes. Its compounds are used to make many things, including fertilizers, water softeners, match heads, and fine china. Antimony is a metalloid, and bismuth is a metal. These elements are added to metals to lower their melting points. Automatic fire sprinkler heads sometimes contain bismuth. The metal melts from the heat of a fire and turns on the sprinkler. The Oxygen Group 8 Copyright © Glencoe/McGraw-Hill, a division of The McGraw-Hill Companies, Inc. Oxygen Group 16 on the O periodic table is the oxygen 16 group. Oxygen, a nonmetal, S makes up about 21 percent 34 of air. Oxygen exists in the Se air as a diatomic molecule, 52 O2. During electrical storms, Te some oxygen molecules, 84 O2, change into ozone, O3. Po Nearly all living things on Earth need O2 to live. Living things also depend on a layer of O3 in the atmosphere, called the ozone layer. The ozone layer protects living things from the Sun’s radiation. Picture This 5. Use a Diagram What is the atomic number of oxygen? Sulfur The second element in the oxygen group is sulfur. It is a nonmetal and has several allotropes. Sulfur combines with metals to form compounds called sulfides. Some sulfides are colorful and are used as pigments in paints. Other Elements Selenium, a nonmetal, and tellurium and polonium, metalloids, are the other elements in Group 16. You need a tiny amount of selenium in your body, so it is included in multivitamins. But too much selenium is toxic. Selenium also is used in photocopiers. Synthetic Elements Scientists have created elements that usually do not exist on Earth. These are called synthetic elements. To create them, scientists smash existing elements with particles from a heavy ion accelerator. Except for some isotopes of natural elements, all synthetic elements have more than 92 protons. Many synthetic elements disintegrate, or fall apart, soon after they are made. It may seem strange to make elements that fall apart, or disintegrate, but scientists are learning about atoms this way. 6. Apply What happens to many of the synthetic elements after they are made? Reading Essentials 309 How are synthetic elements used? Scientists smash protons into uranium to make neptunium, element 93. In about two days, half of neptunium atoms disintegrate. When neptunium atoms disintegrate, they form plutonium, element 94. Plutonium has been produced in nuclear reactors and is used in bombs. Plutonium also can be changed to americium, element 95. You probably have some americium in your home. There is a small amount of the element in smoke detectors. An electric plate in smoke detectors attracts some of the charged americium particles and makes an electric current. A lot of smoke will break the current and set off an alarm. 7. Infer The atomic number of einsteinium is 99. Is einsteinium a transuranium element? Why or why not? 8. Draw Conclusions Why do you think scientists have not discovered new natural elements in many years? 310 What are transuranium elements? Transuranium elements have more than 92 protons, the atomic number of uranium. The transuranium elements are located toward the bottom of the periodic table. Some are in the actinide series. Others are on the bottom row of the main periodic table. All of the transuranium elements 92 are synthetic. They also U are unstable and many disintegrate quickly. Why make elements? For centuries, scientists discovered new natural elements. Now, all new elements are created in laboratories. When these atoms disintegrate, they are said to be radioactive. Radioactive elements can be useful. For example, technetium has medical uses. If most transuranium elements break down quickly, why make them? Scientists make new elements to study the forces that hold the nucleus together. In the 1960s, scientists theorized that stable synthetic elements exist. Maybe scientists will one day find a way to make transuranium elements that do not break down. Perhaps a transuranium element will be found that has everyday uses. CHAPTER 17 Elements and Their Properties Copyright © Glencoe/McGraw-Hill, a division of The McGraw-Hill Companies, Inc. Picture This After You Read Mini Glossary allotropes: different forms of the same element that have different molecular structures metalloids: elements that can form ionic and covalent bonds with other elements and can have the properties of metals and nonmetals semiconductor: an element that can conduct electricity under certain conditions transuranium element: an element with more than 92 protons 1. Review the terms and their definitions in the Mini Glossary. Write a sentence that tells what semiconductors are used for. 2. Use the graphic organizer below to list the properties of metalloids. Then list examples of some important metalloids from each group. Metalloids Copyright © Glencoe/McGraw-Hill, a division of The McGraw-Hill Companies, Inc. Boron Group Carbon Group 3. Oxygen Group Nitrogen Group As you read the section, you highlighted the element groups that were discussed and the names and uses of some of the elements in each group. Was this a good strategy for learning the information? Why or why not? End of Section Reading Essentials 311