Experiment 6: EDTA Titration of the Hardness of Water
advertisement

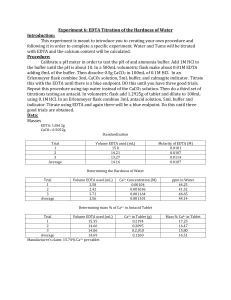
Experiment 6: EDTA Titration of Water Hardness Objective: The purpose of this lab is to design and test and experiment for the hardness (hard=high number of cations) of water. Procedure: Calibrate a pH meter with buffer to confirm the pH of 6M NH3 Buffer Mix HCL and NH3 buffer to a pH of 10 Make a 0.01M EDTA solution using 0.9306g EDTA and 4mL of NH3 buffer in a 250mL volumetric flask Make a CaCl2 solution with 0.5g Ca2+ and 100mL of 0.1M HCl Add 3mL of CaCl2 solution in a 250mL Erlenmeyer flask with 5mL of NH3 buffer and calmagite indicator Titrate with EDTA until Blue for 3 good trials Add 55mL Tap water in a 250mL Erlenmeyer flask with 3mL NH3 buffere and calmagite indicator Titrate with EDTA until Blue for 3 good trials Add 1 crushed TUMS tablet to a 100mL volumetric flask Add 5mL of NH3 buffer and dilute with 0.1M HCl Add 5mL of TUMS solutions to a 250mL Erlenmeyer flask with 2mL of NH3 buffer and calmagite indicator Titrate with EDTA until blue for 3 good trials Data: Standardization of EDTA with CaCO3 Mass EDTA: 0.9307g Mass CaCO3: 0.5023g Trial 1 2 3 Average Titration of Tap Water Trial Water Volume (mL) 1 55.0 2 55.0 3 55.0 Average Volume EDTA (mL) 14.4 14.0 14.8 Volume EDTA (mL) 5.4 5.4 5.7 Molarity EDTA (M) 0.01046 0.01075 0.01017 0.01046 Ca2+ Concentration (M) 0.001027 0.001027 0.001084 0.001046 Ca2+ ppm 41.16 41.16 43.44 41.92 Titration of TUMS Tablet Mass TUMS Tablet: 1.3000g Trial Volume of TUMS Solution (mL) 5.0 5.0 5.0 Average 1 2 3 Volume of EDTA (mL) 4.0 3.7 3.8 Ca2+ in Tablet (g) % Ca2+ in Tablet 0.0321 0.0297 0.0305 0.0308 2.47 2.28 2.35 2.37 Calculations: Title Mass of EDTA Needed Mass of CaCO3 Needed Molarity of EDTA Concentra tion of Ca2+ in tap Water Ca2+ ppm in tap Water Ca2+ in TUMS tablet %Ca2+ in TUMS tablet Formula Calculation 𝐸𝐷𝑇𝐴 = 𝑉𝑤𝑎𝑛𝑡𝑒𝑑 (𝑀𝑜𝑙𝑎𝑟𝑖𝑡𝑦)(𝑀𝑜𝑙𝑎𝑟 𝑀𝑎𝑠𝑠 𝐸𝐷𝑇𝐴) 𝐶𝑎𝐶𝑂3 1𝑚𝑜𝑙 𝐶𝑎𝐶𝑂3 = (𝑀 𝐻𝐶𝑙)(𝑉 𝐻𝐶𝑙) ( ) (𝑀𝑜𝑙𝑎𝑟 𝑀𝑎𝑠𝑠 𝐶𝑎𝐶𝑂3 ) 2𝑚𝑜𝑙 𝐻𝐶𝑙 1 𝑀 𝐸𝐷𝑇𝐴 = 𝑀 𝐶𝑎𝐶𝑂3 (𝑉 𝐶𝑎𝐶𝑂3 ) ( ) 𝑉 𝐸𝐷𝑇𝐴 [𝐶𝑎2+ ] 1𝑚𝑜𝑙 𝐶𝑎2+ 1 = 𝑉 𝐸𝐷𝑇𝐴 (𝑀 𝐸𝐷𝑇𝐴) ( )( ) 1𝑚𝑜𝑙 𝐸𝐷𝑇𝐴 0.055𝐿 𝑊𝑎𝑡𝑒𝑟 0.01𝑚𝑜𝑙 𝐸𝐷𝑇𝐴 = (0.250𝐿) ( ) 𝐿 372.24𝑔 𝐸𝐷𝑇𝐴 ( ) = 0.9306𝑔 𝐸𝐷𝑇𝐴 1𝑚𝑜𝑙 𝐸𝐷𝑇𝐴 1𝑚𝑜𝑙 𝐶𝑎𝐶𝑂3 𝐶𝑎𝐶𝑂3 = (0.100𝑀 𝐻𝐶𝑙)(0.100𝐿 𝐻𝐶𝑙) ( ) 2𝑚𝑜𝑙 𝐻𝐶𝑙 100.09𝑔 ( 𝐶𝑎𝐶𝑂3 ) = 0.5005𝑔 𝐶𝑎2+ 1𝑚𝑜𝑙 𝑀 𝐸𝐷𝑇𝐴 = 0.0502𝑀 𝐶𝑎𝐶𝑂3 (3.0𝑚𝐿 𝐶𝑎𝐶𝑂3 ) 1 ( ) = 0.1046𝑀 14.4𝑚𝐿 𝐸𝐷𝑇𝐴 1𝑚𝑜𝑙 𝐶𝑎2+ [𝐶𝑎2+ ] = 0.0054𝐿 𝐸𝐷𝑇𝐴 (0.01046𝑀 𝐸𝐷𝑇𝐴) ( ) 1𝑚𝑜𝑙 𝐸𝐷𝑇𝐴 1 ( ) = 0.001027 𝑀 0.055𝐿 𝑊𝑎𝑡𝑒𝑟 𝐶𝑎2+ 𝑝𝑝𝑚 = 𝑀 𝐶𝑎2+ (𝑀𝑜𝑙𝑎𝑟 𝑀𝑎𝑠𝑠 𝐶𝑎2+ )(𝐹𝑎𝑐𝑡𝑜𝑟) 40.078𝑔 𝐶𝑎2+ 𝑝𝑝𝑚 = 0.001046𝑀 𝐶𝑎2+ ( 𝐶𝑎2+ ) 1𝑚𝑜𝑙 1000𝑚𝑔 ( ) = 41.92𝑝𝑝𝑚 𝑔 1𝑚𝑜𝑙 𝐶𝑎2+ 𝐶𝑎2+ = 𝑉 𝐸𝐷𝑇𝐴(𝑀 𝐸𝐷𝑇𝐴) ( ) 1𝑚𝑜𝑙 𝐸𝐷𝑇𝐴 2+ (𝑀𝑜𝑙𝑎𝑟 𝑀𝑎𝑠𝑠 𝐶𝑎 )(𝐷𝑖𝑙𝑢𝑡𝑖𝑜𝑛) 𝑚𝑎𝑠𝑠 𝑜𝑓 𝐶𝑎2+ ∗ 100 = % 𝐶𝑎2+ 𝑚𝑎𝑠𝑠 𝑜𝑓 𝑡𝑎𝑏𝑙𝑒𝑡 1𝑚𝑜𝑙 𝐶𝑎2+ 𝐶𝑎2+ = 0.0040𝐿 𝐸𝐷𝑇𝐴(0.01046𝑀 𝐸𝐷𝑇𝐴) ( ) 1𝑚𝑜𝑙 𝐸𝐷𝑇𝐴 40.078𝑔 20 𝑝𝑎𝑟𝑡𝑠 ( 𝐶𝑎2+ ) ( ) = 0.0321𝑔 1𝑚𝑜𝑙 𝑆𝑜𝑙𝑢𝑡𝑖𝑜𝑛 % 𝐶𝑎2+ = 0.0321𝑔 𝐶𝑎2+ ∗ 100 = 2.47% 𝐶𝑎2+ 1.3000𝑔 𝑡𝑎𝑏𝑙𝑒𝑡 Conclusion: The hardness of the tap water as determined to be 41.92ppm. This falls into the soft range of 060ppm described by the United States Geological Service. The Ca2+ content in a TUMS tablet was found to be 2.37%. The value on the container is 15.4%. Our value is significantly lower than expected. A major source of error was determining the end point of the titration. The end color was blue and occasionally the solution would change fully to blue but after a short rest it would revert back to the previous pink color. This would have caused an increase in the amount of EDTA used.