EDTA Titration: Water Hardness Lab Report
advertisement

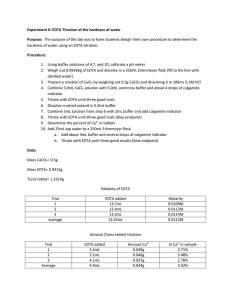
Experiment 6: EDTA Titration of the Hardness of Water Purpose: The purpose of this experiment is to design a procedure for the determination of the hardness of water. Methods: o The pH meter was calibrated with buffer solutions. The pH of 6 M NH4OH ammonia buffer was verified and concentrated HCl was added to reach pH of 10. o A 0.01 M EDTA solution was prepared by massing approximately 0.933 g of EDTA. 4 mL of ammonia buffer was added and diluted to 250 mL using a volumetric flask o A CaCl2 solution was prepared by mixing 0.5005 g CaCO3 with 100 mL of 0.1 M HCl. o 3 mL of CaCl2 solution was placed in a 250 mL Erlenmeyer flask with 5 mL ammonia buffer solution and a small amount of calmagite indicator. The violet/pink solution was titrated with the EDTA solution until the blue endpoint. o This standardization titration was performed two more times. o 25 mL of tap water was pipetted into a 250 mL Erlenmeyer flask. 3 mL ammonia buffer solution and a small amount of indicator were added. This solution was titrated with EDTA solution. o This titration was performed two more times. o One crushed TUMS tablet was placed in a 100 mL volumetric flask. 5 mL ammonia buffer solution was added, and the solution was diluted to the mark with 0.1M HCl. o 5 mL of this solution was placed in a 250 mL volumetric flask with a small amount of buffer and indicator. The solution was then titrated until blue. o This titration was performed two more times. Data: Calculations: Mass EDTA needed for 0.10M solution: Amount CaCO3 needed for CaCl2 solution: Molarity EDTA (Example) Ca+ concentration in Tap Water (Example): Average Hardness of Tap Water: Amount Ca+ in tablet (claim): Amount Ca+ in sample (Example): Conclusion: The purpose of this experiment was to design a procedure for the determination of the hardness of water. A solution was standardized and used to determine the amount of Ca2+ in tap water and TUMS tablets. The hardness of the tap water in the chemistry lab was calculated to be 80.16 ppm, which lies in moderately hard range according to http://www.spectrumwatertech.com/specwaterhard.htm. The average amount of Ca2+ in the TUMS tablet was found to be 24.8 %. This is significantly different than the amount stated by the manufacturer. This could be due to human error in creating the tums solution. The amount stated by the manufacturer is an average so unless more titrations were performed, a direct conclusion cannot be confirmed.