EDTA Titration: Water Hardness & Calcium in Antacids
advertisement

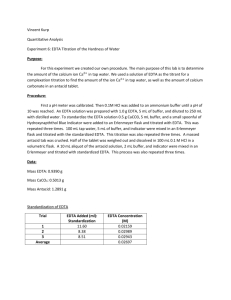
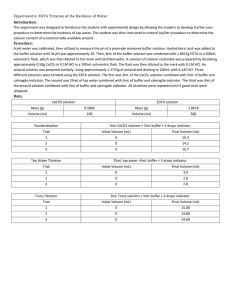
EDTA Titration of the Hardness of Water Purpose: In this experiment one is to design a procedure to find the amount of calcium in unknown samples. This should be a reproducible procedure. Procedure: An EDTA solution with a concentration of 0.1M should be standardized using calcium carbonate. This should take about 1.86g of EDTA in 500mL of distilled water. Make a buffer of 10 pH using NH4 and HCl. Using 20mL of calcium carbonate and 5mL of buffer and an indicator one will do the standardization. The determination of calcium in the water, use 25mL of tap water, 5mL of buffer and indicator titrate with EDTA. Then For the calcium determination for the antacid tablets, make a 100mL solution consisting of 7.5mL solution of HCl, 5mL of buffer and indicator titrate with EDTA. Determine the amount of calcium in tablets, and in the water using collected data. Data: EDTA: 1.8612g CaCO3: 0.2533g CaCO3: 0.00253mol In 250mL Water 0.01012 M CaCO3 Standardization Trial 1 2 3 Average Volume EDTA (mL) 27.70 23.31 26.72 25.91 Molarity of EDTA (M) 0.0091 0.0108 0.0095 0.0098 Hardness of Water Trial 1 2 3 Average Tablet # 1 2 3 Volume EDTA (mL) 6.21 5.00 5.00 5.40 Tablet Mass (g) 1.273 1.255 1.267 Ca2+ Concentration (M) 0.00243 0.00196 0.00196 0.00212 ppm in Water 97.38 78.55 78.55 84.82 Titration only used 0.5g Antacid Tablet Ca2+ in Antacid Tablet Trial Volume EDTA used (mL) 6.72 7.12 5.50 6.45 1 2 3 Average Ca2+ in Tablet (g) Mass % Ca2+ in Tablet 0.1677 0.1752 0.1366 0.1598 13.17 13.96 10.78 12.63 Calculations: Grams of EDTA needed 𝑀𝑜𝑙𝑎𝑟 𝑚𝑎𝑠𝑠 𝐸𝐷𝑇𝐴 𝑀𝑑𝑒𝑠𝑖𝑟𝑒𝑑 × 𝑉𝑜𝑙𝑑𝑒𝑠𝑖𝑟𝑒𝑑 × = 1 𝑚𝑜𝑙 𝐸𝐷𝑇𝐴 𝑔 𝑛𝑒𝑒𝑑𝑒𝑑 Molarity of EDTA Grams of EDTA needed Example 𝑀 𝐶𝑎𝐶𝑂3 ×𝑉𝑜𝑙 𝐶𝑎𝐶𝑂3 𝑉𝑜𝑙 𝐸𝐷𝑇𝐴 0.01012𝑀 𝐶𝑎𝐶𝑂3 ×0.025𝐿 𝐶𝑎𝐶𝑂3 0.010012𝐿 = 𝑀 𝐸𝐷𝑇𝐴 292.14𝑔 𝐸𝐷𝑇𝐴 0.01𝑀 × 0.500𝐿 × = 1.8612𝑔 𝑛𝑒𝑒𝑑𝑒𝑑 1 𝑚𝑜𝑙 𝐸𝐷𝑇𝐴 Molarity of EDTA for Trial 1 = 0.0091𝑀 𝐸𝐷𝑇𝐴 Moles of CaCO3 1 𝑚𝑜𝑙 𝐶𝑎𝐶𝑂3 𝑔𝐶𝑎𝐶𝑂3 × = 𝑚𝑜𝑙 𝐶𝑎𝐶𝑂3 100.087𝑔 Molarity of CaCO3 𝑚𝑜𝑙𝑒𝑠 𝐶𝑎𝐶𝑂3 =𝑀 𝑉𝑜𝑙 Moles of CaCO3 Example Ca2+ in Tap water Ca2+ in Tap water for Trial 1 1 𝑚𝑜𝑙 𝐶𝑎2+ 1 𝑚𝑜𝑙 0.2533𝑔 × 1 𝑚𝑜𝑙 𝐶𝑎𝐶𝑂3 = 0.00253𝑚𝑜𝑙 𝐶𝑎𝐶𝑂3 100.087𝑔 Molarity of CaCO3 Example 0.00253𝑚𝑜𝑙𝑒𝑠 𝐶𝑎𝐶𝑂3 = 0.1012𝑀 𝐶𝑎𝐶𝑂3 0.250𝐿 𝑉𝑜𝑙 𝐸𝐷𝑇𝐴 × 𝑀 𝐸𝐷𝑇𝐴 × × = 1 𝑚𝑜𝑙 𝐸𝐷𝑇𝐴 𝑉𝑜𝑙 𝑤𝑎𝑡𝑒𝑟 𝐶𝑎2+ 𝑐𝑜𝑛𝑐 0.00621𝐿 × 0.0098𝑀 × PPM of Ca2+ PPM of Ca2+ 𝑀𝑜𝑙𝑎𝑟 𝑚𝑎𝑠𝑠 1000𝑚𝑔 𝐶𝑎2+ 𝑀 × × = 𝑝𝑝𝑚 1 𝑚𝑜𝑙 1𝑔 Mass % Ca2+ expected in tablet Mass of Ca+2 in Antacid 𝑀𝑜𝑙𝑎𝑟 𝑀𝑎𝑠𝑠 1 𝑚𝑜𝑙 1 𝑚𝑜𝑙 0.025𝐿 = 0.00243𝑀 40.078𝑔 1000𝑚𝑔 × = 97.38 𝑝𝑝𝑚 1 𝑚𝑜𝑙 1𝑔 0.5𝑔𝐶𝑎𝐶𝑂3 × 1𝑚𝑜𝑙 100.0869𝑔 × 1𝑚𝑜𝑙 𝐶𝑎2+ 1𝑚𝑜𝑙 𝐶𝑎𝐶𝑂3 2+ × 40.078𝑔 1𝑚𝑜𝑙 = 0.20023𝑔 𝐶𝑎2+ 0.20023𝑔 𝐶𝑎 = 15.74% 1.273𝑔 𝑡𝑎𝑏𝑙𝑒𝑡 Mass of Ca+2 in Antacid × 𝑇𝑎𝑏𝑙𝑒𝑡 𝑀𝑎𝑠𝑠 .5𝑔 =𝑔 Percentage of Ca2+ 𝐶𝑎2+ 𝑐𝑎𝑙𝑐𝑢𝑙𝑎𝑡𝑒𝑑 𝑚𝑎𝑠𝑠 𝑜𝑓 𝑚𝑎𝑠𝑠 𝑜𝑓 𝑡𝑎𝑏𝑙𝑒𝑡 × Mass % Ca2+ expected in tablet example 1𝑚𝑜𝑙 1𝑚𝑜𝑙 𝐶𝑎2+ 40.078𝑔 𝑔𝐶𝑎𝐶𝑂3 𝑝𝑒𝑟 𝑡𝑎𝑏 × × × 100.0869𝑔 1𝑚𝑜𝑙 𝐶𝑎𝐶𝑂3 1𝑚𝑜𝑙 𝑔 𝐶𝑎2+ = = 𝑚𝑎𝑠𝑠 % 𝑔 𝑡𝑎𝑏𝑙𝑒𝑡 𝑉𝑜𝑙 𝐸𝐷𝑇𝐴 × 𝑀 𝐸𝐷𝑇𝐴 × 0.00243𝑀 × 1 𝑚𝑜𝑙 𝐶𝑎2+ 1 𝑚𝑜𝑙 𝐸𝐷𝑇𝐴 0.000672 × 0.0098𝑀 × 40.078𝑔 1 𝑚𝑜𝑙 × 1.273𝑔 .5𝑔 = 0.1677𝑔 Percentage of Ca2+ for Trial 1 × 100 = % 0.1677𝑔 × 100 = 13.17% 1.273𝑔 Conclusion: This experiment was to determine the amount of calcium in antacid tablets. Overall this experiment went well, there was an error of about 19%. The calculated calcium percentage in the tablet was 15.7% and the average experimentally was 12.63%. The concentration determined for the tap water was high compared to the results from the York Water Company, which are about 20.30PPM, where we calculated an average of 84.82PPM. But this could be due to error in our experiment due to over titration. Many times the endpoint was not clear. There could have also been errors in the concentrations of solutions.