Experiment 6
advertisement

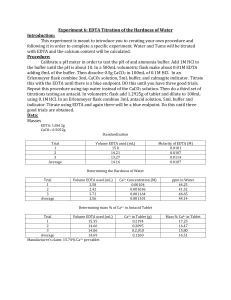
Experiment 6: EDTA Titration of the Hardness of Water Purpose: The purpose of this experiment is to titrate EDTA by your own design. Procedure: 1) A pH meter was calibrated with buffer solution until the pH of 6M ammonia buffer was verified. 2) HCl concentration was added to the ammonia buffer until it reached a pH of approximately 10. 3) A .01M EDTA solution was prepared by massing approx. 0.9306g of EDTA adding 4mL of ammonia buffer and placed it in a 250mL volumetric flask, diluting it to the mark. 4) A calcium chloride solution was prepared by reacting approx. 0.500g of calcium carbonate with 100mL of 0.1M HCl. 5) 3mL of calcium carbonate solution was then placed in a 250mL Erlenmeyer flask. 6) 5mL of ammonia buffer was added and a small amount of calmagite indicator 7) The solution was titrated with EDTA solution until the violet turned a blue color. Need 3 good trials. 8) 50mL of tap water was pipeted into a 250mL Erlenmeyer flask along with 3mL ammonia buffer and a small amount of calmagite indicator. 9) The solution was titrated with EDTA and repeated until 3 good trials were obtained. 10) 1 crushed Tums tablet was massed and placed in a 100mL volumetric flask. 11) 5mL of ammonia buffer was added to the solution and then the solution was diluted with 0.1M HCl to the mark. 12) 5mL of this solution was placed into a 250mL Erlenmeyer flask with approx. 2mL ammonia buffer and a small amount of indicator. 13) The pink solution was then titrated until a blue color appeared. 14) Was repeated until 3 good trials were obtained. Data: Mass of EDTA: .9313g Mass of CaCO3: .5000g Mass of tablet: 1.3214g Trial 1 2 3 Average Molarity of EDTA EDTA added (mL) 13.7 13.4 13.2 13.43 Molarity of EDTA 0.0109 0.0112 0.0114 0.0112 Tap Water Titration EDTA added (mL) Ca2+ concentration (M) 5.1 2.28 x 10-4 6.2 2.78 x 10-4 6.6 2.96 x 10-4 5.97 2.67 x 10-4 Trial 1 2 3 Average Tums Titration EDTA added (mL) 5.5 5.1 4.1 4.9 Trial 1 2 3 Average ppm 9.14 11.14 11.86 10.71 Ca2+ in sample (%) 3.71% 3.48% 2.80% 3.33% Calculations: Grams of EDTA needed molar mass of EDTA disodium × . 01mol × 0.25L = mass of EDTA needed L 372.24g x 0.01M x 0.25L = 0.9306g EDTA mol Molarity of EDTA grams CaCO3 × molar mass CaCO3 × 0.5000gCaCO3 × 0.003L CaCO3 1 × = Molarity of EDTA 0.1L L EDTA added 1mol 0.003LCaCO3 1 × × = 0.0109M EDTA 100.09g CaCO3 0.1L 0.0137L Ca2+ Concentration in Tap Water L EDTA added × M EDTA × 0.0051L × 0.0112M × 1mol Ca2+ 1 × = Molarity of Ca2+ 1mol EDTA . 25L 1mol Ca2+ 1 × = 2.28 × 10−4 M Ca2+ 1mol EDTA . 25L Hardness of Water M Ca2+ × molar mass Ca2+ × 2.28 × 10−4 M x 1000mg = 𝑝𝑝𝑚 1g 40.078g Ca2+ 1000mg x = 9.14𝑝𝑝𝑚 mol 1g Tums Tablet (Claimed) 1 1mol Ca2+ 0.5000g CaCO3 × × × molar mass Ca2+ = grams of Ca2+ molar mass CaCO3 1mol CaCO3 0.5000g CaCO3 x 1mol CaCO3 1mol Ca2+ 40.078g Ca2+ x x = 0.2002g Ca2+ 100.09g CaCO3 1mol CaCO3 1mol 0.2002g x100 = 15.15% 1.3214g Tums Tablet (Experiment) 1mol Ca2+ 20 parts L EDTA added × Mavg EDTA × × molar mass Ca2+ × = grams of Ca2+ 1mol EDTA solution 0.0055L × 0.0112M × 1mol Ca2+ 40.078g 20 parts × × = 0.049g 1mol EDTA 1mol solution 0.049g x 100 = 3.71% 1.3214g Difference: 15.15-3.33 = 11.82% Conclusion: The purpose of this experiment was to determine the hardness of water. The hardness of water for this experiment was determined to be 10.71ppm which is considered to be soft water. The claimed amount of Ca2+ in the Tums tablet was calculated to be 15.15%, which was a large difference compared to the experimental value of an average of 3.33%. There are several errors that could have been present when conducting this experiment which were titrating past the endpoint, or not having clean glassware to collect the tap water.