Intermolecular forces
advertisement

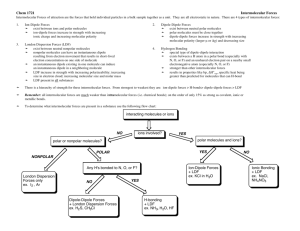
CHM 214: INTERMOLECULAR FORCES AND PHASE CHANGES OF MATTER Why doesn’t everything exist as a gas? Because intermolecular forces (IMF) or interactions keep molecules together gas Gas Liquid Solid shape & volume assumes vol & shape of container assumes shape of container has its own shape & volume compress easy to compress v. difficult to compress diffusion flow molecular order Rapid readily disordered Slow readily disordered v. difficult to compress very slow no ordered KINETIC ENERGY & INTERMOLECULAR FORCES Kinetic energy (KE) prevents molecules from interacting with each other, i.e., KE overcomes attractive intermolecular forces (IMF) Gas: KE IMF Liquid: KE IMF Solid: KE IMF • KE 𝛼 T • Energy IN (heating): solid → liquid → gas • Keep this mind; we’ll be coming back to this during rest of semester! PHASE CHANGES & TEMPERATURE Energy Change: attractions…) ∆Hsub, ∆Hfus, ∆Hvap (preview of coming Intermolecular forces • • Relative strengths of IM forces can be experimentally observed: higher boiling point (or melting point) ⇓ intermolecular forces Electrostatic attraction↑ as intermolecular distances↓; different IMFs have varying dependence on distance • • Strength of IM forces depends on: Q charge on ion μ dipole moment α polarizability polarizability (α): ease with which e– clouds become distorted α as number of e– ↑ α as size (MW) ↑ Types of intermolecular forces depends on ion–ion Q ionic bond Q, μ ion–dipole ions in aqueous solutions of electrolytes (Chap 13) μ dipole–dipole neutral polar molecule interactions Q, α ion-induced dipole ions in nonpolar solvents μ, α dipole-induced dipole polar molecules in nonpolar solvents α dispersion induced-dipole–induced dipole hydrogen bonding directional must have H bonded to N,O,F Electrostatic Forces dipole-dipole draw these Lewis structure & determine ED & molecular geometries and polarity: excellent practice for the exam • for molecules of ~ equal MW & size: more polar molecules (larger μ) have IM forces • Stronger IM forces ⇒ melting point (MP) or boiling point (BP); use MP or BP to compare relative strengths of IMF (just like what we did when comparing lattice energies [ion-ion interactions] in Lecture 8). Dispersion forces • Polarizability (α): ease of electronic distortion; α increases as number of e– ↑; α increases as size (MW) ↑; highly polarizable molecules: more subject to dispersion forces London dispersion forces (LDF) • Observation: nonpolar molecules liquefy, so there must be attractive IM forces present, otherwise it would remain a gas • Electrons are in constant motion (Chap 6) • Dipole moment forms when there are more electrons on one side of the molecule; when this happens, a dipole in a neighboring molecule is induced; flicker in sync • LDF occur with all molecules! (not ionic compounds) LDF (cont.) Ar •••• • Ar H–Cl in addition to ≡ dipole-dipole Cl–H dispersion forces are related to size (primary factor) & shape (secondary factor) • size (number of e– or MW) related to polarizability BLB Table Dispersion interactions (cont.) • • Intermolecular forces ion-dipole dipole-dipole London dispersion forces hydrogen bonds phase diagrams