Ch 11 Intermolecular Forces
advertisement

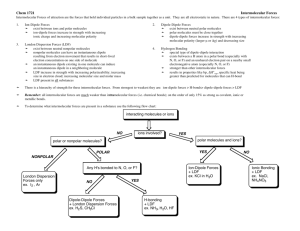
Chapter 11: Intermolecular Forces, Liquids, and Solids Tadas Rimkus Period 2 AP Chemistry Intermolecular Forces Intermolecular forces are the forces that exist between molecules. They include: Ion-dipole forces Dipole-dipole forces London Dispersion forces Hydrogen bonding The last 3 are called van der Waals forces as well Intermolecular Forces The strengths of intermolecular forces vary greatly depending on the substance, but they are generally much weaker that intramolecular forces. http://www.chem.ufl.edu/~itl/2045/matter/FG11_002.GIF Boiling and Melting Point The boiling point of liquids and melting point of solids are dependent on intermolecular forces The higher the temperature at the boiling/melting point, the stronger the forces Ion – Dipole Forces Exist between an ion and the partial charge on the end of a polar molecule Polar molecules are dipoles Ion-dipole forces are important for solutions of ionic substances in polar liquids (NaCl in water) Dipole – Dipole Forces Exist between neutral polar molecules They are effective only when the molecules are very close together and are generally weaker than ion-dipole forces The strength of these forces tends to increase with increasing polarity of the molecules involved London Dispersion Forces Exist between ALL molecules The molecules create an instantaneous dipole that causes the non-polar molecules to attract or repel Like dipole-dipole, these forces are only significant when the molecules are very close together Tends to increase with increasing molecular weight London Dispersion Forces Polarizability is the ease with which the electrons in a molecule can be distorted (the “squashiness” of the electron cloud) More polarizable molecules have stronger London dispersion forces London Dispersion Forces Pentane bp=309.4 K http://genchml ab.union.edu/c hem101_110_i ntermolecular_ forces/npentane.jpg Neopentane bp=282.7 K http://uploa d.wikimedia. org/wikipedi a/commons/ b/b4/Neope ntane-3Dballs.png The shape of the molecules also affects the strength of the dispersion force The larger the surface area of the molecule, the stronger its dispersion force Comparing Intermolecular Forces When the molecule have similar molecular weights and shapes, dispersion forces are basically equal. In this case, the differences are due to dipole-dipole attractions, with the most polar molecules having the strongest attractions. When molecules vary greatly in their molecular weights, dispersion forces tend to be the decisive forces. Hydrogen Bonding Hydrogen bonding is a special type of intermolecular force that exists between the hydrogen atom in a polar bond and a fluorine, oxygen, or nitrogen atom. H-F, H-O, H-N It is the strongest of the intermolecular forces Distinguishing Between Forces http://itl.chem.ufl.edu/2041_f97/matter/FG11_012.GIF Phase Changes http://www.alterniawhatif.com/HPS%20Project/Phase%20Chan ges_files/phase_change.jpg Heating Curves Heating curves incorporate the phase diagram but also show the energy required for the phase change http://library.thinkquest.org/C006669/media/Chem/img/Gra phs/HeatCool.gif Critical Temperature and Pressure The critical temperate is the highest temperature at which a substance can exist as a liquid. Critical pressure is the pressure required to liquefy the substance at this critical temperature The greater the intermolecular forces, the higher the critical temperature and the more easily is liquefies Phase Diagrams D D B A C http://ltl.tkk.fi/research/theory/TypicalPD.gif Phase Diagrams A phase diagram is a visual used to explain the conditions under which equilibria exist between the different states of matter The line from A to B is the liquid vapor-pressure curve It ends at the critical point (the critical temperature and pressure of the substance) Beyond this point, the liquid and gas phases are indistinguishable (supercritical fluid) Phase Diagrams The line from A to C represents the vapor pressure of the solid as it sublimes at different temperatures The line from A to D represents the change in melting point of the solid with increasing pressure Point A is known as the triple point. All 3 phases are at equilibrium at this temperature and pressure Phase Diagrams of H2O and CO2 http: //ww w.cb u.ed u/~ mco ndre n/wa terphas ediagr am.j pg http: //ww w.te amo nslau ght.f snet. co.u k/co 2%2 0pha se% 20di agra m.GI F The phase diagram of carbon dioxide (right) follows the typical behavior, with its melting point increasing with increasing pressure The phase diagram of water (left) shows that the melting point decreases with increasing pressure