interacting molecules or ions ions involved? polar molecules and
advertisement

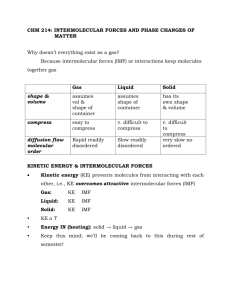
Chem 1721 Intermolecular Forces Intermolecular Forces of attraction are the forces that hold individual particles in a bulk sample together as a unit. They are all electrostatic in nature. There are 4 types of intermolecular forces: 1. Ion-Dipole Forces ➛ exist between ions and polar molecules ➛ ion-dipole forces increase in strength with increasing ionic charge and increasing molecular polarity 3. London Dispersion Forces (LDF) ➛ exist between neutral nonpolar molecules ➛ nonpolar molecules can have an instantaneous dipole resulting from electron movement that results in short-lived electron concentration on one side of molecule ➛ an instantaneous dipole existing in one molecule can induce an instantaneous dipole in a neighboring molecule ➛ LDF increase in strength with increasing polarizeability; increasing size in electron cloud; increasing molecular size and molar mass ➛ LDF present in all substances 2. Dipole-Dipole Forces ➛ exist between neutral polar molecules ➛ polar molecules must be close together ➛ dipole-dipole forces increase in strength with increasing molecular polarity (larger µ or Δχ) and decreasing size 4. Hydrogen Bonding ➛ special type of dipole-dipole interaction ➛ exists between a H atom in a polar bond (especially with N, O, or F) and an unshared electron pair on a nearby small electronegative atom (especially N, O, or F) ➛ stronger than other intermolecular forces ➛ results in properties like bp, ΔH°vap, specific heat being greater than predicted for molecules that can H-bond ➛ There is a hierarchy of strength for these intermolecular forces. From strongest to weakest they are: ion-dipole forces > H-bonds> dipole-dipole forces > LDF ➛ Remember: all intermolecular forces are much weaker than intramolecular forces (i.e. chemical bonds); on the order of only 15% as strong as covalent, ionic or metallic bonds. ➛ To determine what intermolecular forces are present in a substance use the following flow chart: interacting molecules or ions NO ions involved? YES polar molecules and ions? polar or nonpolar molecules? YES POLAR NO NONPOLAR Any H's bonded to N, O, or F? London Dispersion Forces only ex. I 2 , Ar NO Dipole-Dipole Forces + London Dispersion Forces ex. H2 S, CH3Cl Ion-Dipole Forces + LDF ex. KCl in H2O YES H-bonding + LDF ex. NH 3, H2O, HF Ionic Bonding + LDF ex. NaCl, NH4 NO3