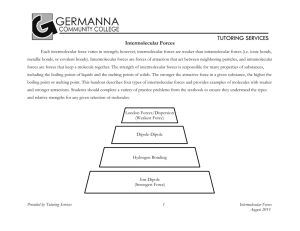
IMF_TA_Generated
advertisement
Intermolecular Forces and Phase Change by Kim Law and Alex Shames! (and Mr. Bennett) How do geckos stick to things? Which intermolecular force is this? http://www.youtube.com/watch?v=H Tbrsx1zARs (Start at 0:40) Gecko feet have a large surface area because of all the hairs. Which IMF increases with surface area? Scientists made a substance to mimic the properties of the gecko’s foot. What kind of properties should their material have? Which structure matches which liquid? Oil Honey Hint: Think about each liquid’s consistency. Is it slow moving or fast moving? Viscosity Resistance to deformation Commonly thought of as thickness of liquids More IMF = more viscous (think honey vs. water) Why does sweating cool us off? This marathon runner is ridiculously photogenic-probably because he’s staying nice and cool thanks to sweating. Why is that? How come this salad dressing separated? The olive oil and vinegar clearly don’t mix. Why can’t they just get along? Here’s the structure of olive oil Here’s the structure of vinegar Why does ice cream melt on a hot summer day? M&M’s don’t melt on a hot summer day don’t melt. What does this mean about the intermolecular forces in each material? How does this spider walk on water? Would the spider be able to do this on oil? Why or why not? Here’s he structure of oil again as a reminder. Surface Tension Resistance to external force. Stronger IMF = greater surface tension http://ga.water.usgs.gov/edu/gra phics/surfacetension-diagram.gif The End Now be ready for a pop quiz! Shout out to Katie Shi