8.4 Intermolecular forces
advertisement

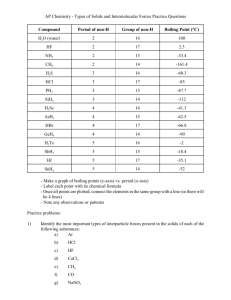
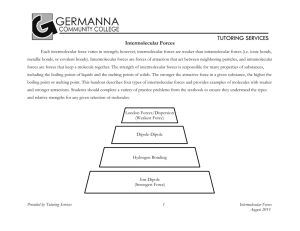
Attractions Between Molecules or Intermolecular Forces (IM Forces) Chapter 8.4 Learning Objectives • Understand the difference between intramolecular and intermolecular forces • Identify the 4 common types of intermolecular forces We have already talked about intramolecular forces. Intramolecular forces hold atoms together in a molecule. Covalent bonds would be an example. Intermolecular forces are attractive forces between molecules. Intermolecular Forces Intramolecular = strong Intermolecular = weak They do control physical properties such as boiling and melting points, vapor pressure, and viscosity Types of Intermolecular Forces Van der Waals Forces • Dipole-dipole interactions • Ion-Dipole interactions • London dispersion forces Hydrogen bonding Dipole-Dipole Interactions Molecules that have permanent dipoles are attracted to each other. Ion-Dipole Interactions Attractive forces between an ion and a polar molecule Ion-Dipole Interaction The larger the charge the stronger the force London Dispersion Forces Occasionally electrons wind up on the same side of the atom. London Dispersion Forces At that instant, the helium atom is polar. London Dispersion Forces This polar helium atom then induces a dipole on a neighboring helium atom. London Dispersion Forces London dispersion forces, or dispersion forces, are attractions between an instantaneous dipole and an induced dipole. London Dispersion Forces • These forces are present in all molecules, whether they are polar or nonpolar. • The tendency of an electron cloud to distort in this way is called polarizability. Types of Intermolecular Forces Hydrogen Bonding • The dipole-dipole interactions experienced when H is bonded to N, O, or F are unusually strong. • We call these interactions hydrogen bonds. Hydrogen Bonding: Water Hydrogen Bonding: Water