CHUnit 14 with 5E and LEP- FINAL (6-27-08)
advertisement

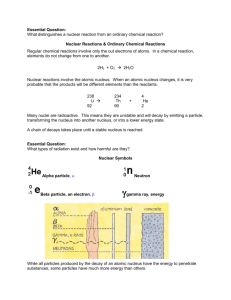
I. Grade Level/Unit Number: 9-12 Unit 14 II: Unit Title: Nuclear Chemistry III. Unit Length: 4 days (on a 90 min. per day block schedule) IV. Major Learning Outcomes: Students should be able to: Nuclear Chemistry V. Use the symbols for and distinguish between alpha (42He), and beta (-10e) nuclear particles, and gamma (γ) radiation include relative mass.. Use shorthand notation of particles involved in nuclear equations to balance and solve for unknowns. Example: The neutron is represented by (01n). Discuss the penetrating ability of alpha, beta, and gamma radiation. Conceptually describe nuclear decay, including: o Decay as a random event, independent of other energy influences o Using symbols to represent simple balanced decay equations o Half-life (including simple calculations). Contrast fission and fusion. Cite illustrations of the uses of nuclear energy, including, but not limited to: electricity, carbon-14 dating, and radioisotopes for medicine (tracers, ionizing radiation, gamma sterilization, etc). Content Objectives Includes (with RBT Tags): Objective Objective Number 4.03 Analyze nuclear energy. Radioactivity: characteristics of alpha, beta and gamma radiation. Decay equations for alpha and beta emission. Half-life. Fission and fusion. RBT Tag C4 VI. English Language Development Objectives (ELD) Included: NC English Language Proficiency (ELP) Standard 4 (2008) for Limited English Proficiency Students (LEP)- English Language learners communicate information, ideas, and concepts necessary for academic success in the content area of science. Suggestions for modified instruction and scaffolding for LEP students and/or students who need additional support are embedded in the unit plan and/or are added at the end of the corresponding section of the lessons. The amount of scaffolding needed will depend on the level of English proficiency of each LEP student. Therefore, novice level Chemistry- Unit 14 1 DRAFT students will need more support with the language needed to understand and demonstrate the acquisition of concepts than intermediate or advanced students. VII. Materials/Equipment Needed Activity Determining & Graphing the Half-Life of Twizzlers Graphical Representation of Half-Life: Determining & Graphing the Half-Life of M & Mium Materials Twizzlers Graph paper M & Ms Graph paper White paper Plastic cups VIII. Detailed Content Description: Please see the detailed content description for each objective in the chemistry support document. The link to this downloadable document is in the Chemistry Standard Course of Study at: http://www.ncpublicschools.org/curriculum/science/scos/2004/24chemistry IX. Unit Notes: This unit is focused on the concept of nuclear chemistry. Students should be able to distinguish between alpha, beta, and gamma radiation and compare penetrating abilities. They will learn how to balance nuclear equations and solve unknowns. Students will distinguish between fission and fusion and be able to describe modern uses of nuclear energy. In each unit, Goal 1 objectives which relate to the process of scientific investigation are included. In each of the units, students will be practicing the processes of science: observing, hypothesizing, collecting data, analyzing, and concluding. The Goal 1 Objectives are as follows: COMPETENCY GOAL 1: The learner will develop abilities necessary to do and understand scientific inquiry. 1.01 Design, conduct and analyze investigations to answer questions related to chemistry. Identify questions and suggest hypotheses. Identify variables. Use a control when appropriate. Select and use appropriate measurement tools. Collect and organize data in tables, charts and graphs. Chemistry- Unit 14 This goal and these objectives are an integral part of each of the other goals. In order to measure and investigate scientific phenomena, students must be given the opportunity to design and conduct their own investigations in a safe laboratory. The students should 2 DRAFT Analyze and interpret data. Explain observations. Make inferences and predictions. Explain the relationship between evidence and explanation. Identify how scientists share findings. use questions and models to formulate the relationship identified in their investigations and then report and share those finding with others Students will be able to: Identify questions and suggest hypotheses. Identify variables. Use a control when appropriate. Select and use appropriate measurement tools. Collect and organize data in tables, charts and graphs. Analyze and interpret data. Explain observations. Make inferences and predictions. Use questions and models to determine the relationships between variables in investigations. Identify how scientists share findings. If a teacher follows this curriculum (s)he will have addressed the goals and objectives of the SCOS. However, teachers may want to substitute other activities that teach the same concept. The unit length has extra time built in for quizzes, going over homework, additional practice depending on the nature of the class, and assessment. Teachers should utilize the textbook as a resource by assigning homework each day and providing additional guided and independent practice. Reference Tables: The North Carolina Chemistry Reference Tables were developed to provide essential information that should be used on a regular basis by students, therefore eliminating the need for memorization. It is suggested that a copy be provided to each student on the first day of instruction. A copy of the reference tables can be downloaded at the following URL: http://www.ncpublicschools.org/docs/curriculum/science/scos/2004/chemistry/referencet ables.pdf Chemistry- Unit 14 3 DRAFT Essential Questions: Essential questions for this unit are embedded within the unit. Essential questions are those questions that lead to student understanding. Students should be able to answer these questions at the end of an activity. Teachers are advised to put these questions up in a prominent place in the classroom. The questions can be answered in a journal format as a closure. Safety: Students should wear chemical splash goggles during any lab activity involving chemicals. This includes household substances. It is extremely important for the safety and success of your students that you do ALL activities and labs prior to assigning them to students. At the beginning of each lab, the teacher should address any specific safety concerns relating to the activity. Computer Based Activities: Several of the recommended activities are computer based and require students to visit various internet sites and view animations of various biological processes. These animations require various players and plug-ins which may or may not already be installed on your computers. Additionally some districts have firewalls that block downloading these types of files. Before assigning these activities to students it is essential for the teacher to try them on the computers that the students will use and to consult with the technology or media specialist if there are issues. These animations also have sound. Teachers may wish to provide headphones if possible. X. Global Content: Aligned with 21st Skills One of the goals of the unit plans is to provide strategies that will enable educators to develop the 21st Century skills for their students. As much as students need to master the NCSOS goals and objectives, they need to master the skills that develop problem solving strategies, as well as the creativity and innovative thinking skills that have become critical in today’s increasingly interconnected workforce and society. The Partnership for 21st Century Skills website is provided below for more information about the skills and resources related to the 21st Century classroom. http://www.21stcenturyskills.org/index.php?option=com_content&task=view&id=27&Ite mid=120 NC SCS Chemistry 1.01 & 4.03 21st Century Skills Communication Skills Conveying thought or opinions effectively Activity 1.01 & 4.03 Nuclear Equation Problems Half Life Problems When presenting information, distinguishing between relevant and irrelevant information Chemistry- Unit 14 4 DRAFT 1.01 & 4.03 Explaining a concept to others 1.01 & 4.03 1.01 & 4.03 1.01 & 4.03 1.01 & 4.03 1.01 & 4.03 1.01 & 4.03 1.01 & 4.03 4.03 1.01 & 4.03 4.03 Interviewing others or being interviewed Computer Knowledge Using word-processing and database programs Developing visual aides for presentations Using a computer for communication Learning new software programs Employability Skills Assuming responsibility for own learning Persisting until job is completed Working independently Developing career interest/goals Responding to criticism or questions Information-retrieval Skills Searching for information via the computer Searching for print information Searching for information using community members Language Skills - Reading Following written directions Identifying cause and effect relationships Summarizing main points after reading Nuclear Equation Problems Half Life Problems All activities Nuclear Equation Problems Half Life Problems Most of the activities can be presented as opportunities for students to follow written directions. The teacher will have to work with most students to develop this skill over time. The following activities are well suited to developing skills in following directions: Nuclear Equation Problems Half Life Problems Nuclear Equation Problems Half Life Problems Locating and choosing appropriate reference materials Reading for personal learning Chemistry- Unit 14 5 DRAFT 1.01 & 4.03 1.01 & 4.03 1.01 & 4.03 1.01 & 4.03 1.01 & 4.03 Language Skill - Writing Using language accurately Organizing and relating ideas when writing Proofing and Editing Synthesizing information from several sources Documenting sources Developing an outline Writing to persuade or justify a position Creating memos, letters, other forms of correspondence Teamwork Taking initiative Working on a team Thinking/Problem-Solving Skills Identifying key problems or questions Evaluating results Developing strategies to address problems Developing an action plan or timeline All activities ENGAGE: (30 minutes) Determining & Graphing the Half-Life of Twizzlers This activity will engage students as they are introduced to the term half-life and how it is related to radioactive elements. Students will do part the M&Mium activity later in the unit. This activity was developed through a project called the Science Behind our Food which was supported by a grant through the National Science Foundation. Please see the link below: http://www.uga.edu/discover/sbof/ Essential Question: How are half-life related to a radioactive element? Chemistry- Unit 14 6 DRAFT Chemistry- Unit 14 7 DRAFT Chemistry- Unit 14 8 DRAFT EXPLAIN: Have students to present their answers and graphs from the Half-Life of Twizzlers activity to the class. Instruct them to explain their reasoning. ELABORATE: (10 minutes) Nuclear Reactions The teacher will begin the discussion by explaining the difference between nuclear reactions with ordinary chemical reactions. Explain that the nucleus of a radioactive element spontaneously decomposes. Essential Question: What distinguishes a nuclear reaction from an ordinary chemical reaction? Language (ELP) Objectives for LEP Students: In written paragraph or chart form, compare and contrast nuclear reactions and chemical reactions using key vocabulary: radioactive, elements, isotopes, decomposition, atoms, etc. Verbally share conclusions with partner and or class. Nuclear Reactions & Ordinary Chemical Reactions Regular chemical reactions involve only the out electrons of atoms. In a chemical reaction, elements do not change from one to another. 2H2 + O2 2H2O Nuclear reactions involve the atomic nucleus. When an atomic nucleus changes, it is very probable that the products will be different elements than the reactants. 238 U 92 4 234 Th + 90 He 2 Chemistry- Unit 14 9 DRAFT Many nuclei are radioactive. This means they are unstable and will decay by emitting a particle, transforming the nucleus into another nucleus, or into a lower energy state. A chain of decays takes place until a stable nucleus is reached. ELABORATE: (10 minutes) Nuclear Symbols The teacher will explain the nuclear symbols: alpha, beta, neutron and gamma. The penetrating ability should be compared. Essential Question: What types of radiation exist and how harmful are they? Language (ELP) Objectives for LEP Students: Define key vocabulary: alpha, beta, neutron, and gamma. How do they relate to the study of nuclear chemistry? In written form, discuss the essential question on the types of radiation and how they are harmful. Nuclear Symbols Alpha particle, Beta particle, an electron, Neutron Chemistry- Unit 14 10 DRAFT While all particles produced by the decay of an atomic nucleus have the energy to penetrate substances, some particles have much more energy than others. ELABORATE: (20 minutes) Balancing Nuclear Equations The teacher should begin the discussion with a review of the superscript/subscript isotopic notation. The teacher should explain how to balance nuclear equations. o Explain that when writing a nuclear equation, the sums of the mass numbers and atomic numbers of the reactants must equal the sums of the mass and atomic numbers of the products. o Students should check their equations by comparing the sums of the superscripts and subscripts on each side of the equation. Essential Question: How is a nuclear equation balanced? EVALUATE: (30 minutes) Balancing Nuclear Equations This activity will allow students to assess their understanding of balancing nuclear equations with guided and independent practice. After students have completed these problems, the teacher will evaluate students’ understanding by going over the problems with the students. Essential Question: How would you balance a nuclear equation? Balancing Nuclear Equations Practice Chemistry- Unit 14 11 DRAFT 1. Complete and balance these nuclear equations by supplying the missing particles: a) 6629Cu 6630Zn + _____ b) 0-1e + _____ 73Li c) 2713Al + 42He 3014Si + _____ d) 8537Rb + _____ 8235Br + 42He 2. Strontium-90 Has half-life of 28 years. If a 1.00-mg sample was stored for 112 years. What mass of Sr-90 would remain? 3. Write nuclear equation for the alpha decay of (a) 19278Pt (b) 21084Po 4. Write nuclear equations for the beta decay of (a) 23993Np (b) 9038Sr 5. Determine the type of emission or emissions (alpha, beta, or gamma) that occurred in the following transitions: (a) 21082Pb to 210 82Pb (b) 23491Pa to 23089Ac to 23090Th (c) 23490Th to 23088Ra to 23088Ra 6. Complete and balance these nuclear equations by supplying the missing particles: (a) 2713Al + 42He 3015P + _____ (b) 2714Si 0+1e + _____ (c) _____ + 21H 137N + 10n (d) _____ 8236Kr + 0-1e 7. Strontium-90 has half-life of 28 years. If a sample was tested in 1980 and found to be emitting 240 counts/ min, in what year would the same sample be found to be emitting 30counts/min? How much of the original Sr-90 would be left? Chemistry- Unit 14 12 DRAFT Radioactivity Match the three types of emission with the following ideas. Each may be used once, more than once, or not at all. a) alpha b) beta c) gamma ___ 1. Two protons and two neutrons ___ 2. High speed electron ___ 3. ___ 4. 42 He ___ 5. Higher energy than x-rays ___ 6. ___ 7. Helium nucleus ___ 8. 0 1 e Complete the following nuclear equations: 59 1 56 1. 27 Co + 0 n 25 Mn + 2. 14 6 C 3. 99 42 Mo + 4. 235 92 5. 40 19 14 7 N + 0 1 e + U + K + 40 18 0 0 (alpha decay) Ar EXPLORE: (45 minutes) Graphical Representation of Half-Life: Determining & Graphing the Half-Life of M & Mium In this activity, students will explore how the half-life of a radioactive element is determined and how to represent half-life graphically. Chemistry- Unit 14 13 DRAFT Essential question: How is the half-life of a radioactive element determined and graphically represented? Chemistry- Unit 14 14 DRAFT Chemistry- Unit 14 15 DRAFT Chemistry- Unit 14 16 DRAFT Chemistry- Unit 14 17 DRAFT EXPLAIN: Have students to present their answers and graphs from the Half-Life of M & Mium activity to the class. Instruct them to explain their reasoning. ELABORATE: (20 minutes) Half Life The teacher will introduce the term “half’-life” and explain to students how it is related to radioactive elements. Essential Question: How is the half-life of a radioactive element used to determine how much of a sample is left after a given period of time? Language (ELP) Objectives for LEP Students: Verbally or in written form, describe the concept of half-life and explain how it is graphically represented. Half-Life The length of time it takes for one-half of the atoms of a radioactive nuclide to disintegrate. Half-Life Table Nuclide Half-life Decay Type 6 2 He 0.802 seconds Beta-minus 1.3 minutes Alpha and Gamma 12.3 years Beta-minus 5730 years Beta-minus 227 92 U 3 1 H 14 6 C 235 92 U 7.1 x 10 8 years Alpha and Gamma Chemistry- Unit 14 18 DRAFT Many radioactive particles decay into other radioactive particles, however, the final product of radioactive decay will always be a stable substance. Uranium-238 goes through a long sequence of decays before it finally becomes stable. The 14 individual decays that lead from U-238 to Pb206 are shown here. How many alpha particles are released? How many beta particles? are released? Chemistry- Unit 14 19 DRAFT EVALUATE: (30 minutes) Calculating Half-Life This activity will allow students to assess their understanding of half-life calculations with guided and independent practice. After students have completed these problems, the teacher will evaluate students’ understanding by going over the problems with the students. HALF-LIFE PROBLEMS 1. An isotope of cesium (cesium-137) has a half-life of 30 years. If 1.0 mg of cesium-137 disintegrates over a period of 90 years, how many mg of cesium-137 would remain? 2. A 2.5 gram sample of an isotope of strontium-90 was formed in a 1960 explosion of an atomic bomb at Johnson Island in the Pacific Test Site. The half-life of strontium-90 is 28 years. In what year will only 0.625 grams of this strontium-90 remain? 3. Actinium-226 has a half-life of 29 hours. If 100 mg of actinium-226 disintegrates over a period of 58 hours, how many mg of actinium-226 will remain? 4. Thallium-201 has a half-life of 73 hours. If 4.0 mg of thallium-201 disintegrates over a period of 6.0 days and 2 hours, how many mg of thallium-201 will remain? 5. The half-life of isotope X is 2.0 years. How many years would it take for a 4.0 mg sample of X to decay and have only 0.50 mg of it remain? 6. Selenium-83 has a half-life of 25.0 minutes. How many minutes would it take for a 10.0 mg sample to decay and have only 1.25 mg of it remain? 7. Element-106 has a half-life of 0.90 seconds. If one million atoms of it were prepared, how many atoms would remain after 4.5 seconds? 8. Three grams of Bismuth-218 decay to 0.375 grams in one hour. What is the halflife of this isotope? HALF LIFE GRAPHS Chemistry- Unit 14 20 DRAFT 1. What is the half life of the graphed material? ____ 2. What mass of radioisotope will remain after 9.0 hours? _____ 3. Plot the data from a substance with a half-life of 1.5 hours. HALF LIFE PROBLEMS 1. Lr-257 has a half life of 8 seconds. What % of a sample will remain 32 seconds after it is made? 2. Na-24 has a half life of 15 hours. How long will it take for a sample to decay to 25% of its original mass? 3. A 64 gram sample of I-131 is tested after 40 days and is found to contain only 2 grams of I-131. What is the half life of I-131? ELABORATE: Comparing Fission & Fusion (10 minutes) The teacher will discuss the differences between fission reactions with fusion reactions. o Fission: heavy nuclei are split into lighter nuclei. Fission reactions are relatively easy to control but produce radioactive wastes. o Fusion: light nuclei are combined to form heavier nuclei. Fusion reactions are difficult to initiate and control but produce little radioactive wastes. Essential Question: How are fission reactions different from fusion reactions? Chemistry- Unit 14 21 DRAFT Language (ELP) Objectives for LEP Students: In paragraph form or chart form, compare and contrast fission reactions and fusion reactions. Give specific examples. Fission Chemistry- Unit 14 22 DRAFT Fusion Chemistry- Unit 14 23 DRAFT ELABORATE: Uses of Nuclear Energy (30 minutes) Students will use their textbooks or the internet to research uses of nuclear energy, including, but not limited to: electricity, carbon-14 dating and radioisotopes for medical uses. Essential Question: What are some modern uses of nuclear energy? EXPLAIN: (20 minutes) Following the research activity, have students describe various uses of Nuclear Energy. EVALUATE: Sample Assessment Questions for Unit 14: Unit Goal/ RBT Tag 14 Questions 4.04 C4 1. Determine the half-life of Iron-59, an isotope used to diagnose blood disorders, from the following data? Elapsed Time (days) Mass remaining 0 2.00 g 22.25 1.41g 44.50 1.00g 89.00 0.500g a. 22.25 days b. 44.50 days c. 89.00 days d 156.0 days 2. When carbon-14 decays, it emits a beta particle to produce nitrogen14, as shown below. Chemistry- Unit 14 24 DRAFT 3. When copper-67 undergoes beta decay, which of the following isotopes is produced? A. copper-66 B. copper-68 C. nickel-67 D. zinc-67 4. Which radioactive emission requires the most shielding? a. positrons b. gamma rays c. beta particles d. alpha particles 5. The given reaction: 2713Al + a. beta decay. b. artificial transmutation. c. fission. d. fusion. 4 He 2 31 P 15 best represents EVALUATE: (45 minutes) Below are sample test items obtained from the WIZARD test bank developed by eduware™ that can be used to allow students to assess their understanding and abilities and allow the teacher to evaluate the students understanding of key concepts and skill development for this unit. Chemistry- Unit 14 25 DRAFT Chemistry- Unit 14 26 DRAFT Chemistry- Unit 14 27 DRAFT Chemistry- Unit 14 28 DRAFT Chemistry- Unit 14 29 DRAFT