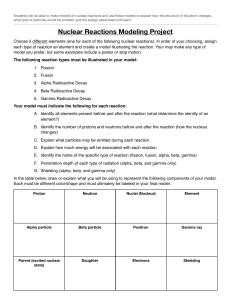
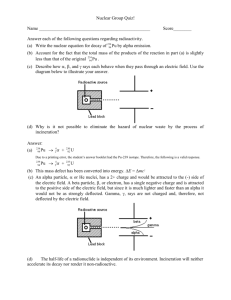
Nuclear Equations Worksheet: Alpha & Beta Decay
advertisement

Nuclear Equations Worksheet 1. Nuclei are often written in the form i) A Z X . What does the letter A represent? ____________________________________________________________ ii) What does the letter Z represent? ____________________________________________________________ iii) Iron (Fe) has an atomic number 26 and a mass number of 56. Write down the nuclear formula for iron. ____________________________________________________________ 2. Write down the nuclear symbols for alpha particles and beta particles. Alpha particle _________________ Beta particle ________________ 3. What is meant by the term ‘nuclear decay’? _______________________________________________________________ 4. Aluminium can be written as i) 27 13 Al . How many protons are in the aluminium nucleus? ____________________________________________________________ ii) How many neutrons are in the aluminium nucleus? ____________________________________________________________ iii) How many electrons are there in a neutral aluminium atom? ____________________________________________________________ 5. Radon-222 decays into Polonium-218. Complete the nuclear equation by inserting the correct numbers in the blank spaces. 222 86 Rn __ __ Po 24 6. Lead-210 decay by emitting a beta particle. Complete the nuclear equation by inserting the correct numbers in the blank spaces. 210 82 Pb __ __ Bi 10