Nuclear Chemistry video notes
advertisement

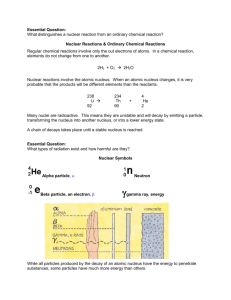
Nuclear Chemistry Project – Chapter 14 Part I. Watch the following video and complete the questions on a separate sheet of paper. http://teachertube.com/viewVideo.php?video_id=48485 1. 2. 3. 4. 5. 6. 7. 8. 9. List 4 differences between a chemical reaction and a nuclear reaction. How does the rate of a chemical reaction compare to the rate of a nuclear reaction? Name the 3 most common types of radiation. List the symbol associated with each type. Give another name for an alpha particle. What symbol can we use for this? What blocks alpha particles? Give another name for a beta particle. What symbol can we use for this? What blocks beta particles? How is a gamma ray different from alpha and beta particles? What is the only way to block gamma radiation? **You may skip the section on deflection.** Complete the following table. You may try to do it before watching the video or you may fill it in as you watch. Name Short hand Charge Symbol How to Energy Make up block Alpha Beta Gamma 10. Describe how to write the formula for an isotope. 11. Write the reaction for radium-226 converting into radon-222. 12. What type of decay is this reaction? 13. Write the reaction of carbon-14 turning into nitrogen-14. 14. What type of decay is this reaction? 15. Write the reaction of uranium-238 undergoing alpha and gamma decay. 16. What is half life? 17. What unit is half life measured in? 18. You have 100 g of an isotope. How much is left after 1 half life? 19. How much will be left after 2 half lives? 20. What is fission? 21. What do we use fission reactions for? 22. What is fusion? 23. Why don’t we use fusion reactions for power? 24. Where do fusion reactions occur? Part II. Watch the following video on balancing nuclear equations: http://teachertube.com/viewVideo.php?video_id=139478 25. Write the equation for uranium-238 spontaneously decays by emitting an alpha particle. Part III. Review the notes you received in class, and complete the following questions: 1. Complete and balance these nuclear equations by supplying the missing particles: a) 6629Cu 6630Zn + _____ b) 0-1e + _____ 73Li c) 2713Al + 42He 3014Si + _____ d) 8537Rb + _____ 8235Br + 42He 2. Write nuclear equation for the alpha decay of (a) 19278Pt (b) 21084Po 3. Write nuclear equations for the beta decay of (a) 23993Np (b) 9038Sr 4. Match the three types of emission with the following ideas. Each may be used once, more than once, or not at all. a) alpha b) beta c) gamma d) neutron ___ 1. Two protons and two neutrons ___ 2. High speed electron ___ 3. Most penetrating power ___ 4. 42 He ___ 5. Higher energy than x-rays ___ 6. Must be stored in a lead container ___ 7. Helium nucleus ___ 8. 0 1 e Write the nuclear equation for each of the following: 1. 238 92 U undergoes alpha decay 2. Lead-210 emits a beta particle. 3. Thorium-230 decays by emission of an alpha particle and a gamma ray. 4. 27 13Al + 42He 3015P + _____ 5. 27 14Si 0+1e + _____ 6. _____ + 21H 137N + 10n 7. _____ 8236Kr + 0-1e Complete the following half life problems. Show your work and circle your final answer. 1. An isotope of cesium (cesium-137) has a half-life of 30 years. If 1.0 mg of cesium-137 disintegrates over a period of 90 years, how many mg of cesium-137 would remain? 2. Actinium-226 has a half-life of 29 hours. If 100 mg of actinium-226 disintegrates over a period of 58 hours, how many mg of actinium-226 will remain? 3. Thallium-201 has a half-life of 73 hours. If 4.0 mg of thallium-201 disintegrates over a period of 6.0 days and 2 hours, how many mg of thallium-201 will remain? 4. The half-life of isotope X is 2.0 years. How many years would it take for a 4.0 mg sample of X to decay and have only 0.50 mg of it remain? 5. Selenium-83 has a half-life of 25.0 minutes. How many minutes would it take for a 10.0 mg sample to decay and have only 1.25 mg of it remain? Essential Question: What distinguishes a nuclear reaction from an ordinary chemical reaction? Nuclear Reactions & Ordinary Chemical Reactions Regular chemical reactions involve only the out electrons of atoms. In a chemical reaction, elements do not change from one to another. 2H2 + O2 2H2O Nuclear reactions involve the atomic nucleus. When an atomic nucleus changes, it is very probable that the products will be different elements than the reactants. 238 U 92 234 Th 90 + 4 He 2 Many nuclei are radioactive. This means they are unstable and will decay by emitting a particle, transforming the nucleus into another nucleus, or into a lower energy state. A chain of decays takes place until a stable nucleus is reached. Essential Question: What types of radiation exist and how harmful are they? Nuclear Symbols Alpha particle, Beta particle, an electron, Neutron gamma ray, energy While all particles produced by the decay of an atomic nucleus have the energy to penetrate substances, some particles have much more energy than others. Essential Question: How is a nuclear equation balanced? o Example 1: 238 234 4 Th + He U→ 92 90 2 There are two important things to note here: The sum of the masses (top numbers) are equal on both sides of the equation. [238 =234 + 4] The sum of the atomic numbers (the bottom numbers) also are equal on both sides of the equation. [92 = 90 + 2] o Example 2: 14 14 N+ C→ 6 0 7 e -1 Again, the top and bottom numbers are equal on both sides of the equation ([14 = 14 + 0] and [6 = 7 + 1]). Essential Question: How is the half-life of a radioactive element used to determine how much of a sample is left after a given period of time? Half-Life The length of time it takes for one-half of the atoms of a radioactive nuclide to disintegrate. Notice that some half-lives are short, while others are very long. Many radioactive particles decay into other radioactive particles. However, the final product of radioactive decay will always be a stable substance. Half-Life Table Nuclide Half-life Decay Type 6 2 He 0.802 seconds Beta-minus 1.3 minutes Alpha and Gamma 5730 years Beta-minus 227 92 U 14 6 C 235 92 U 7.1 x 10 8 years Alpha and Gamma Essential Question: How are fission reactions different from fusion reactions? o Fission: heavy nuclei are split into lighter nuclei. Fission reactions are relatively easy to control but produce radioactive wastes. Fission o Fusion: light nuclei are combined to form heavier nuclei. Fusion reactions are difficult to initiate and control but produce little radioactive wastes. Fusion Essential Question: What are some modern uses of nuclear reactions? Medicine Electricity, Power Carbon-14 dating