218 final - Marshall University Personal Web Pages
advertisement

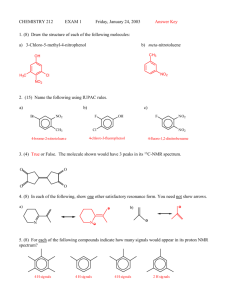
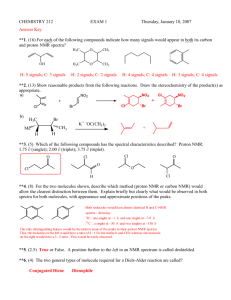
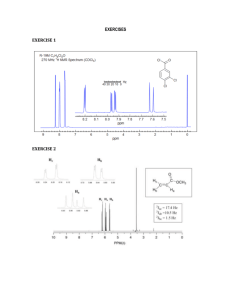
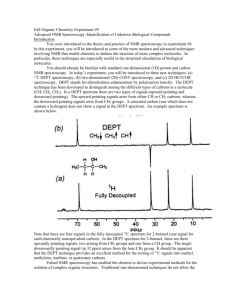
Nmre ___________________________ 1. (5 pts) Match the C-13 spectra below to the small alcohols: ______ CH3CH2 CH2OH 1-propanol ______ (CH3)2CHOH iso propyl alcohol ______ (CH3)2CH CH2OH iso butyl alcohol Spectrum A all peaks equal size 2. (10 pts) Determine the structure of both of the following compounds. Indicate what peaks correspond to which carbons in the structure. Molecular chemical shifts (dept Formula results, intensity) u-up n- not there d-down three results from bottom to top on dept spectra. a) C5H11Cl 22(unu,2) 26(uuu,1) 42(und,1) 43(und,1) b) C4H8O2 161(uuu,1) 67(uuu,1) 22(unu,2) Spectrum B right peak twice height of left Spectrum C right peak twice height of others 1 3. (44 points-4 pts each) Short answers. ix. At what temperature is the electromagnet kept at? (include your units) i) On a 60 MHz instrument, 0.2 ppm in the proton spectrum corresponds to _______ Hz. On the same instrument C-13 is run at a frequency of 15 MHz. On its spectrum, 0.2 ppm corresponds to __________ Hz. ii) . 1H has a spin of _____ and ____ orientations where as 2H has a spin of ____ and ____ orientations. x. The signal to noise ratio on a certain instrument is 15:1 when run on a 5 % solution with 16 scans. On the same instrument how many scans will it take to get a signal to noise ratio of 45:1 on a 1 % solution of the same compound? Show your work. iii-vi. Consider the NMR of 2 methyl-2- butanol. iii. Draw the structure of the compound. xi. Consider the proton spectrum of an ethyl group. The methylene group, CH2, has a total intensity of 20. What is the total intensity of the methyl group, CH3, and what would be the intensity of each line in the spectrum? iv) The proton spectrum will consist from high to low chemical shift: a) a singlet, and a multiplet (splitting greater than 4), a singlet and a triplet b) a quartet, and a doublet and a singlet c) a doublet, a multiplet. and a doublet d) a triplet, a singlet, and a doublet e) a singlet, a quartet, a singlet, and a triplet v. The integration of the peaks from high to low chemical shift will be: a) 1:2:6:3 b) 1:1::9:2 c) 2:1:6 d) 4:3:2 e) 1:2:6 vi. The decoupled C-13 spectrum will consist from high to low chemical shift of: a) 5 peaks of equal intensity. b) 4 peaks with one of the middle peaks double the intensity of the other three. c) 5 peaks with one of the middle peaks double the intensity of the other four. d) none of these vii. On the Unity plus 500 NMR in S-152, what is the H1 frequency? (include your units) viii. On the Unity plus 500 NMR in S-152, what is the C-13 frequency? (include your units) 2 3