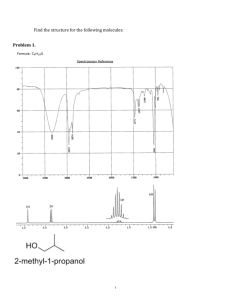
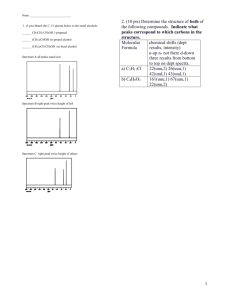
NMR Spectroscopy Exercises: Structure Elucidation
advertisement
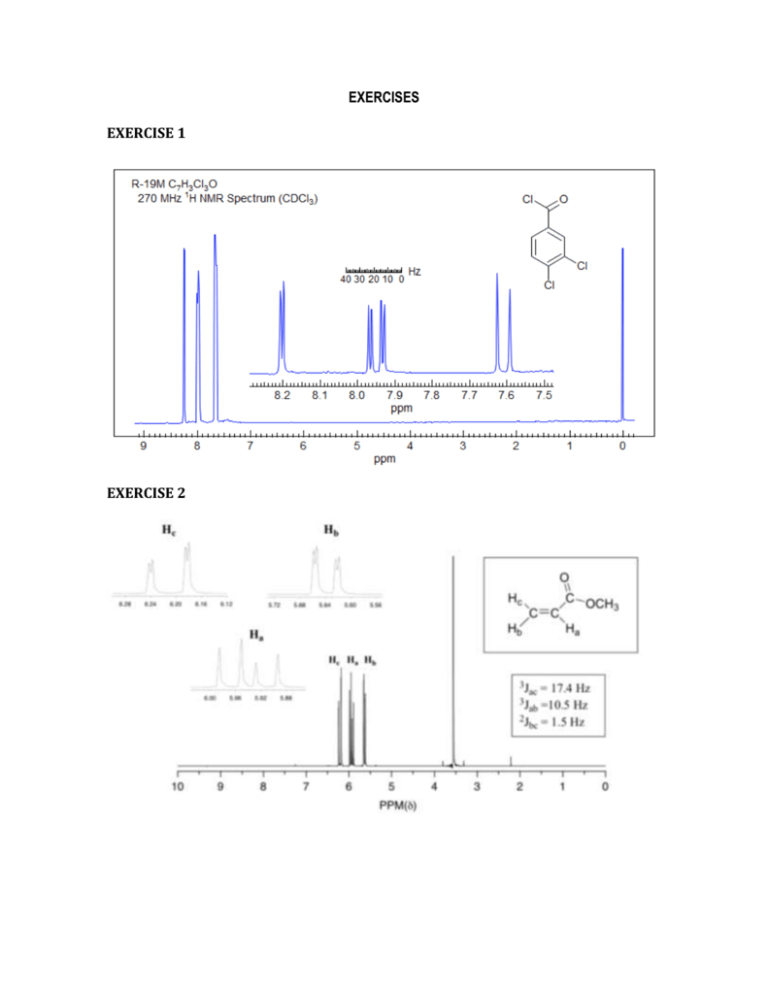
EXERCISES EXERCISE 1 EXERCISE 2 EXERCISE 3 A compound with molecular formula C3H6O2 gives the following peaks in its proton nmr spectrum: Chemical shift Splitting 1.1 2.2 11.8 Triplet Quartet Singlet Integration factor 3 2 1 Identify the molecule and account for the chemical shifts, splitting and integration factors of all three peaks. EXERCISE 4 A compound with molecular formula C5H10O2 gives the following peaks in its proton nmr spectrum: a) Chemical shift Splitting 1.2 1.3 2.3 4.1 Triplet Triplet Quartet Quartet Integration factor 3 3 2 2 Identify the molecule and account for the chemical shifts EXERCISE 5– Combined spectral analysis A compound containing 58.8% carbon, 9.8% hydrogen and 31.4% oxygen is subjected to mass spectrometry and found to give intense peaks at m/z = 43 and m/z = 57, in addition to a molecular ion peak at m/z = 102. Infra-red analysis of the molecule showed a sharp peak at m/z = 1710 cm-1. A proton nmr spectrum of the molecule yielded the following peaks: Chemical shift Splitting 0.8 1.1 2.3 3.7 Triplet Sextet Triplet Singlet Integration factor 3 2 2 3 Deduce the structure of the molecule and account for the formation of all the peaks the spectra. EXERCISE 6 The two spectra below are of phenol and benzaldehyde. Assign them. EXERCISE 7 For each spectrum below, chose between the alternative compounds. Give your reasons. EXERCISE 8 The spectra below are of acetone, 1,2-dichloroethane, 1,1,2-trichloroethane, 2,2dimethoxypropane, 1- bromopropane and 2-bromopropane. Assign them. EXERCISE 9 Assign the following spectra to one of the compounds listed: 1,4-dimethylbenzene, 1,4dimethoxybenzene, phenylethyne, 3-methyl-3-hydroxy-1-butyne, 2-bromobutane, 1,2dibromo-2-methylpropane. EXERCISE 10. Propose plausible structures for the five compounds whose proton NMR spectra are shown: a) C4H10O2; b) C7H7Br; c) C4H9Br; d) C8H9Br EXERCISE 11 Compound Z, C9H12, gives a proton NMR spectrum as shown below. Assign a structure. EXERCISE 12 Two spectra are given below along with their molecular formulas. Propose a structure that corresponds to each spectrum. EXERCISE 13. The integrated 1H NMR spectrum of a compound of formula C4H10O is shown. Propose a structure consistent with the data. EXERCISE 14 The compound whose proton NMR spectrum is shown below has the molecular formula C3H6Br2. Propose a plausible structure. EXERCISE 15 A compound (C10H12O2), whose spectrum appears below was isolated from a reaction mixture containing 2-phenylethnaol and acetic acid. Propose a structure for this compound. EXERCISE 16 The compound whose proton NMR spectrum is shown has the molecular formula C4H7O2Cl and shows an infrared absorption peak at 1740 cm^-1. Propose a plausible structure. EXERCISE 17 A small plant was adding bromine across the double bond of 2-butene to make 2,3dibromobutane. A controller malfunction and allowed the reaction temperature to rise beyond safe limits. A careful distallation of the product showed that several impurities had formed, including the one whose NMR is shown below. Determine the structure. EXERCISE 18 When 2-chloro-2-methylbutane is treated with a variety of strong bases, the products always seem contain two isomers (A and B) of formula C5H10. When sodium hydroxide is used as the base isomer B predominates. Determine the structures of A and B and explain the experimental results.