Exam 1 Key - Chemistry
advertisement

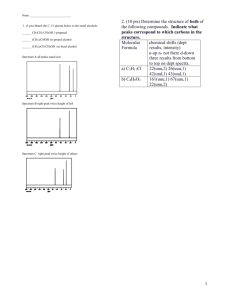
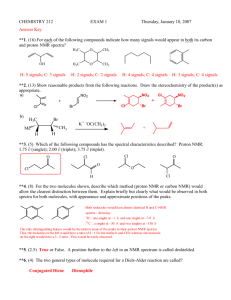
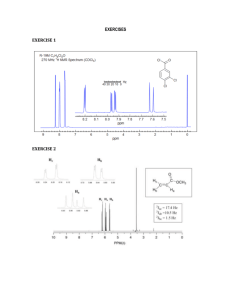
CHEMISTRY 212 EXAM 1 Friday, January 24, 2003 Answer Key 1. (8) Draw the structure of each of the following molecules: a) 3-Chloro-5-methyl-4-nitrophenol b) meta-nitrotoluene CH3 OH H3C NO2 Cl NO2 2. (15) Name the following using IUPAC rules. a) b) Br c) NO2 F CH3 Cl 4-bromo-2-nitrotoluene OH F NO2 NO2 4-chloro-3-fluorophenol 4-fluoro-1,2-dinitrobenzene 3. (4) True or False. The molecule shown would have 3 peaks in its 13C-NMR spectrum. O O O O 4. (8) In each of the following, show one other satisfactory resonance form. You need not show arrows. a) b) N N 5. (8) For each of the following compounds indicate how many signals would appear in its proton NMR spectrum? 4 H sig nals 4 H sig nals 4 H sig nals 2 H sig nals 6. (4.) What splitting pattern (singlet, doublet, etc.) is observed in the 1H-NMR spectrum for the indicated hydrogen? CH3 H3C CH a) doublet d) quintet CH CH2 CH3 H3C b) triplet c) quartet e) sextet CH2 7. (20) Show reasonable products from the following reactions. Draw the stereochemistry of the product(s) as appropriate. a) KNH2 Cl + H3 C b) + Br Cl + F F Cl Br H3C + H3C Br F c) Et Br Et Me KOMe Me Et Me CH2 Et + Me H Cl Et H Et Me Et Me Me CHCH3 + H 8. (12) For each of the following compounds, indicate by yes or no whether or not they contain conjugated systems. a) b) NH c) d) O No Yes O Yes Yes NH 9. (15) For each of the following molecules, indicate the number of different types of hydrogen and the number of different types of carbon present. a) O b) H O 3 different H 3 different C OH c) H 5 different H 5 different C 3 different H 3 different C OH 10. (9) State, in each of the following, whether or not the dienes shown would undergo the Diels-Alder reaction. Me a) b) c) Me Et No Yes No 11. (5) In the Diels-Alder reaction below, which starting material, A, B, C or D, is required to form the product shown? O O A. H A-D? B. O H O C. O D. O 12. (5) The molecule shown would have which 1H-NMR spectrum ? O A. B. C. D. Singlets at ~7 & ~2.5, a quartet at ~2 & a triplet at ~1 A singlet at ~7.5 , a quartet at ~1.5 & a triplet at ~4 A singlet at ~1.75 , a quartet at ~4 & a triplet at ~1.5 A singlet at ~7 , quartets at ~2 & 4& a triplet at ~1.5 13. (7) Peaks in the NMR spectrum which are further to the left are considered to be (downfield, upfield, sinister) and their locations are measured relative to a standard, namely The , TMS . standard should be [circle all which apply]: unreactive, high boiling, a molecule with many identical hydrogens, a symmetrical molecule. 14. (6) Benzene is (more, less, equally) stable than a typical alkene because its double bonds are (circular, conjugal, conjugated). If a benzene ring bears 3 substituents it is called tri substituted and if they are attached 1,4- to one another this would be (ortho, meta, para) substitution. 15. (12) From among the compounds shown below, choose the one that: a) Gives three groups of peaks in its 1H-NMR spectrum; b) Gives the simplest 13C-NMR spectrum; c) Has 3 peaks in its 13C-NMR spectrum. Cl Cl Cl Cl Cl 3 peaks in proton NMR Cl 3 peaks in carbon NMR s implest carbon NMR 16. (3) A very broad, medium strength peak at ~3300-2400 cm-1 in an IR spectrum would indicate A. An amine B. An aromatic ring C. A cyclohexene D. A carboxylic acid E. A ketone 17. (3) There are two key general areas in an infrared spectrum - name them. Functional group and Fingerprint 18. (3) A strong peak at ~2900 cm-1 in an IR spectrum would indicate ? A. A carboxylic acid B. An alkane C. An aromatic ring D. An alkene E. A ketone 19. (3) Sharp peaks at ~3300 cm-1 in an IR spectrum would indicate ? A. A carboxylic acid B. An amine C. An aromatic ring D. An alcohol E. An alkane