Tutorial 3
advertisement

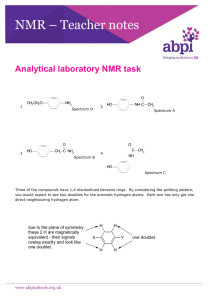
Tutorial 3 NMR The proton nmr of 2-bromo-2-methylpropane will consist of... three quartets and a singlet two doublets and a singlet two singlets one singlet The proton nmr of 2-bromopropane will consist of... two doublets and a sextet a doublet and a septet a singlet, a doublet and a triplet a singlet, a doublet and a triplet The splitting pattern for a signal is found by... -counting the number of chemically equivalent hydrogen atoms on adjacent atoms -counting the number of chemically different hydrogen atoms on adjacent atoms -counting the number of chemically different hydrogen atoms on adjacent atoms and adding 1 -counting the number of chemically different hydrogen atoms on adjacent atoms and subtracting 1 Which one of the following statements about protons on O-H groups is incorrect? -they always produce a doublet -they always produce a singlet -they are not taken into account when working out splitting patterns of adjacent protons -their signal can be removed from its normal location by shaking with deuterium oxide In a triplet, the relative peak areas are in the ratio... 1:1:1 1:2:1 1:3:1 1:4:1 Which compound has a molecular ion at m/z = 58, an infra red absorption at 1650 cm-1 and just one singlet in its nmr spectrum? butane CH3COCH3 CH3CH2CHO 2-methylpropane Which one of the following hydrocarbons produces an nmr spectrum with more than one peak? methane ethane butane cyclobutane The isomer of C4H8 which produces an nmr spectrum with four different signals is... CH2=CHCH2CH3 CH3CH=CHCH3 (CH3)2C=CH2 cyclobutane Which one of the following methods would be best for finding the identity of an organic compound? finding the m/z value of the molecular ion in its mass spectrum its proton nmr spectrum comparing its infra red spectrum with known examples measuring its melting point