Technique Experiment 1 - 1 Chemistry 335. Technique Experiment
advertisement

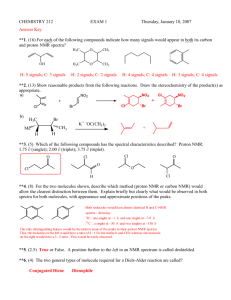
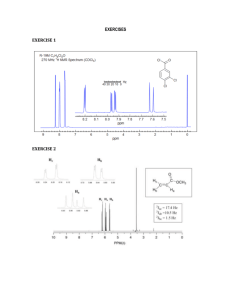
Chemistry 335. Technique Experiment 1A: Separation of Benzoin and Benzil Name:_____________________________________ Date: ____________________ Benzoin 1. Physical Properties (5 points) Color Rf TLC Mobile Phase Amount isolated (mg) Mass recovery (%) 2. IR Spectrum (6 points) Fill in the table below and attach your IR spectrum with peaks labeled. Functional Group IR Absorption (cm-­‐1) List peaks C=O C-­‐O C=C C-­‐H aromatic or vinylic C-­‐H aliphatic Other? Other? 3. 1H NMR Spectrum (11 points) NMR solvent:____________________ 3a. Draw the structure of benzoin and label each distinct proton with a number (3 points). Technique Experiment 1 - 1 3b. Fill in the table below and attach your 1H NMR spectrum with peaks fully labeled. Interpret the chemical shift, splitting pattern, and integration of each peak to make each assignment. Use the numbers in 3a as your assignments in the table below (8 points). Chemical shift Coupling Integration Assignment (doublet, t riplet, e tc) (ppm) 4. Explain in detail the reasoning behind your identification of benzoin. Include a discussion of consistent data and any contradictory data that you decided was not important. (3 points.) Technique Experiment 1 - 2 Benzil 1. Physical Properties (5 points) Color Rf TLC Mobile Phase Amount isolated (mg) Mass recovery (%) 2. IR Spectrum (6 points) Fill in the table below and attach your IR spectrum with peaks labeled. Functional Group IR Absorption (cm-­‐1) List peaks C=O C-­‐O C=C C-­‐H aromatic or vinylic C-­‐H aliphatic Other? Other? 3. 1H NMR Spectrum (11 points) NMR solvent:____________________ 3a. Draw the structure of benzil and label each distinct proton with a number (3 points). Technique Experiment 1 - 3 3b. Fill in the table below and attach your 1H NMR spectrum with peaks fully labeled. Interpret the chemical shift, splitting pattern, and integration of each peak to make each assignment. Use the numbers in 3a as your assignments in the table below (8 points). Chemical shift Coupling Integration Assignment (doublet, t riplet, e tc) (ppm) 4. Explain in detail the reasoning behind your identification of benzil. Include a discussion of consistent data and any contradictory data that you decided was not important. (3 points.) Technique Experiment 1 - 4 Chemistry 335. Technique Experiment 1B: Separation of Two Unknowns Name:_____________________________________ Date: ____________________ Unknown Mixture #:____________ (50 points total) First Compound Isolated off of Column 1. Physical Properties (5 points) Color Rf TLC Mobile Phase Amount isolated (mg) Mass recovery (%) 2. IR Spectrum (6 points) Fill in the table below and attach your IR spectrum with peaks labeled. Functional Group IR Absorption (cm-­‐1) List peaks C=O C-­‐O C=C C-­‐H aromatic or vinylic C-­‐H aliphatic Other? Other? 3. 1H NMR Spectrum (11 points) NMR solvent:____________________ 3a. Draw the structure of your first unknown and label each distinct proton with a number (3 points). Technique Experiment 1 - 1 3b. Fill in the table below and attach your 1H NMR spectrum with peaks fully labeled. Interpret the chemical shift, splitting pattern, and integration of each peak to make each assignment. Use the numbers in 3a as your assignments in the table below (8 points). Chemical shift Coupling Integration Assignment (doublet, triplet, etc) (ppm) 4. Explain in detail the reasoning behind your identification of your first unknown. Include a discussion of consistent data and any contradictory data that you decided was not important. (3 points.) Technique Experiment 1 - 2 Second Compound Isolated off of Column 1. Physical Properties (5 points) Color Rf TLC Mobile Phase Amount isolated (mg) Mass recovery (%) 2. IR Spectrum (6 points) Fill in the table below and attach your IR spectrum with peaks labeled. Functional Group IR Absorption (cm-­‐1) List peaks C=O C-­‐O C=C C-­‐H aromatic or vinylic C-­‐H aliphatic Other? Other? 3. 1H NMR Spectrum (11 points) NMR solvent:____________________ 3a. Draw the structure of your second unknown and label each distinct proton with a number (3 points). Technique Experiment 1 - 3 3b. Fill in the table below and attach your 1H NMR spectrum with peaks fully labeled. Interpret the chemical shift, splitting pattern, and integration of each peak to make each assignment. Use the numbers in 3a as your assignments in the table below (8 points). Chemical shift Coupling Integration Assignment (doublet, t riplet, e tc) (ppm) 4. Explain in detail the reasoning behind your identification of your second unknown. Include a discussion of consistent data and any contradictory data that you decided was not important. (3 points.) Technique Experiment 1 - 4