Isotopes and % Occurrence:
advertisement

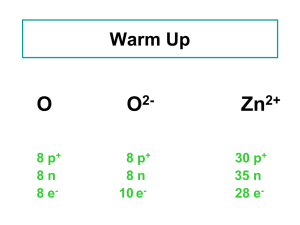
Isotopes and % Occurrence: Calculating the average atomic mass of an element having two or more isotopes Name_________________________ Date_____________ Block____ Isotopes are atoms of the SAME element that have different numbers of neutrons and therefore different mass numbers. Because the atoms are of the same element, each of the atoms has the same number of protons. Each isotope occurs as a percentage of the total number of atoms of the element that exist. For example, the element boron has two isotopes: boron-10 (B-10) and boron-11 (B-11). Both isotopes have atoms that have 5 protons (the atomic number of boron is 5.) The mass number of one of the isotopes is “10.” This means that atoms of that isotope of boron have 5 neutrons in the nucleus. Remember that mass number is the total number of protons and neutrons in the nucleus of an atom. Since there are 5 protons also in the nucleus, the mass number for this isotope is 10. The other boron isotope has a mass number of “11.” This means that this isotope of boron has atoms that contain 6 neutrons in the nucleus. There are also 5 protons in each nucleus, so that the 5 protons added to the 6 neutrons equals a mass number of 11. The isotope B-10 has a relative abundance of 20.0%. This means that 20.0% of all boron atoms have a mass number of 10 (5 protons and 5 neutrons in the nucleus of each atom.) The isotope B-11 has a relative abundance of 80.0%. This means that 80.0% of all boron atoms have a mass number of 11 (5 protons and 6 neutrons in the nucleus of each atom.) To calculate the average atomic mass of the element boron means to calculate the average mass of all of the atoms of boron. The mass of each isotope is multiplied by its relative abundance expressed as a decimal. Remember, “percent” means as so many parts per 100 parts total. The unit for atomic mass is the atomic mass unit (amu). 20.0% = 0.200 80.0% = 0.800 10B 10. amu = 2.0 amu 11B 11 amu x 0.800 = Total average mass = 8.8 amu 10.8 amu x 0.200 The average atomic mass of an atom of boron is 10.8 amu. Note: the decimal point after the ‘10’ means that both the 1 and the 0 are significant. Calculate the average atomic masses for each of the following elements. 1. There are two isotopes of helium: He-3, and He-4. The mass of each of the He-3 atoms is 3.0160 amu. The mass of each of the He-4 atoms is 4.0026 amu. He-3 has a natural relative abundance of 0.000 1%. He-4 has a natural relative abundance of 99.9999 amu. 2. Oxygen has three isotopes: O-16, O-17, and O-18. The relative abundance of O-16 is 99.759%. The relative abundance of O-17 is 0.037%. The relative abundance of O-18 is 0.204%. The measured mass of an atom of O-16 is 15.995 amu. The measured mass of an atom of O-17 is 16.995 amu. The mass of an atom of O-18 is 17.999 amu. 3. Zinc has five isotopes; the data are given in the table below. Masses have been rounded to 3 decimal places; percentages have been rounded to 2 decimal places. Isotope Zn-64 Zn-66 Zn-67 Zn-68 Zn-70 Mass (amu) 63.929 65.926 66.927 67.925 69.925 Natural Relative Abundance (%) 48.89 27.81 4.11 18.57 0.62 4. Element X has two naturally occurring isotopes. An atom of one isotope has a mass of 14.003 amu and has a relative abundance of 99.63%. An atom of the second isotope has a mass of 15.000 amu and has a relative abundance of 0.37%. After calculating the average atomic mass of the element, identify the element by name and symbol.