Biological Molecules: Structure and Function Lecture Notes
advertisement

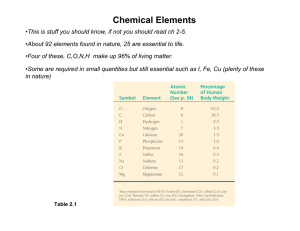
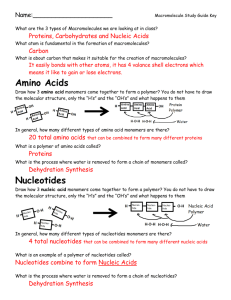
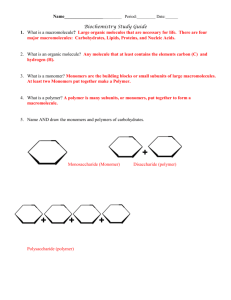
Chapter 5 :Structure and Function of Large Biological Molecules Type of Chemical reactions involved 1. Condensation/Dehydration synthesis reaction- bond between to organic monomers and H2O is released and a polymer is formed 2. Hydrolysis reaction- breaking the bonds between the members of the polymer, H2O is added or required for the reaction. A. Carbohydrates- CHO usually 2x as many H as C and O. 1. Monomers a. 3-5 C with O and H, in a chain or a ring form b. examples: c. check the linear to ring form, Fig. 5.4 d. functions: energy and building block/monomer 2. Polymers a. disaccharides, trisaccarides and polysaccarides b. examples, sucrose and maltose c. functions: i.energy storage, examples ii. structural: cellulose, peptidoglycan, chitin B. Lipids-often a glycerol + chains of fatty acids 1. Fats a. glycerol + 3 fatty acids see Fig. 5.11 b. saturated fat c. unsaturated fat trans fats- arise from the hydrogenating d. functions: 2. Phospholipids a. structure b. function 3. Steroids a. structure, Fig. 5.15 function: C. Proteins/polypeptide 1. Monomers a. amino acids, structure, common structure Fig. 5.17 2. Polymers-the peptide bonds 3. Levels of Protein structure/organization a. primary b. secondary i. alpha helix ii beta pleated sheet c. tertiary d. quaternary 4. Functions: see table 5.1!!! D. Nucleic Acids 1. building block- nucleotide a. Nitrogenous bases- A, T, G, C purines pyrimidines b. PO4 c. monosaccharide- ribose or deoxyribose, Fig. 5.27 2. DNA- polymer that stores hereditary information a. double helix b. complementary c. nucleus or nuclear area, forms chromosomes 3. RNA- polymer that transmits hereditary info into proteins to do the cellular work a. single stranded b. ribose, U replaces T