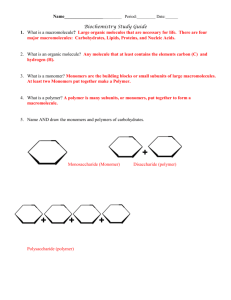
Amino Acids Nucleotides
advertisement
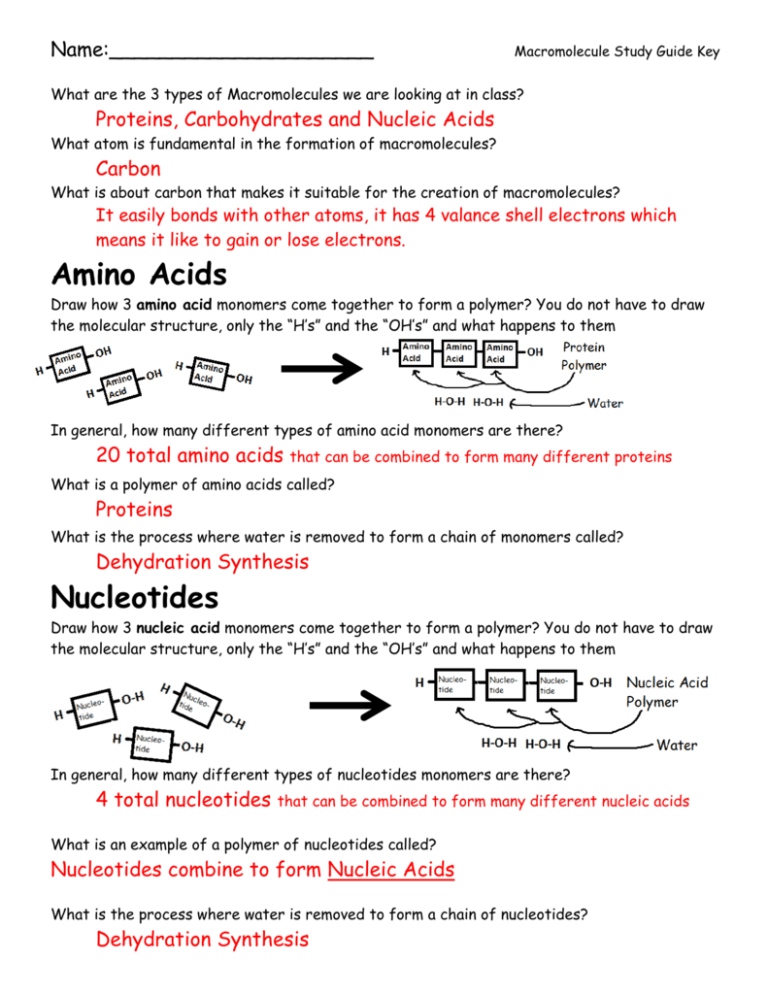
Name:_____________________ Macromolecule Study Guide Key What are the 3 types of Macromolecules we are looking at in class? Proteins, Carbohydrates and Nucleic Acids What atom is fundamental in the formation of macromolecules? Carbon What is about carbon that makes it suitable for the creation of macromolecules? It easily bonds with other atoms, it has 4 valance shell electrons which means it like to gain or lose electrons. Amino Acids Draw how 3 amino acid monomers come together to form a polymer? You do not have to draw the molecular structure, only the “H’s” and the “OH’s” and what happens to them In general, how many different types of amino acid monomers are there? 20 total amino acids that can be combined to form many different proteins What is a polymer of amino acids called? Proteins What is the process where water is removed to form a chain of monomers called? Dehydration Synthesis Nucleotides Draw how 3 nucleic acid monomers come together to form a polymer? You do not have to draw the molecular structure, only the “H’s” and the “OH’s” and what happens to them In general, how many different types of nucleotides monomers are there? 4 total nucleotides that can be combined to form many different nucleic acids What is an example of a polymer of nucleotides called? Nucleotides combine to form Nucleic Acids What is the process where water is removed to form a chain of nucleotides? Dehydration Synthesis Simple Sugars Draw how 3 simple sugar monomers come together to form a polymer? You do not have to draw the molecular structure, only the “H’s” and the “OH’s” and what happens to them What is one example of a simple sugar monomer? Glucose What is a polymer of simple sugars called? Simple sugars combine to form Carbohydrates Overall: What is hydrolysis and how would it work? Explain using one of the polymer chains above and draw a diagram of it. Hydrolysis is when you “add water” to break apart the monomers. Why does carbon like to not only give electrons, but share them? Explain this using the octet rule and comparing it to oxygen, which has a total of 6 electrons in its valance shell Carbon has 4 valance shell electrons and because of the octet rule, which says all atoms want either 0 or 8 electrons in their outer most shell, carbon is willing to give or receive electrons when it shares them. Oxygen (and many other atoms) on the other hand, is only willing to gain two (to go from 68) Using words, explain what is the difference between hydrolysis and dehydration synthesis? Dehydration synthesis is when you take away water from a monomer to make a polymer while hydrolysis is when you add water to go from a polymer to a monomer.