pubdoc_1_9317_348
advertisement

Lecture : 5
BONDING FORCES AND ENERGIES:
As the atoms approach, each exerts forces on the other. These forces
are of two types, attractive and repulsive, and the magnitude of each is
a function of the separation or interatomic distance. The origin of an
attractive force FA depends on the particular type of bonding that exists
between the two atoms. Its magnitude varies with the distance, as
represented schematically in Figure below:
FIGURE : (a) The dependence of repulsive, attractive, and net forces on interatomic separation fortwo isolated toms.
(b) The dependence of repulsive, attractive, and net potential energies on interatomic separation for two
isolated atoms.
The net force FN between the two atoms is just the sum of both
attractive
and repulsive components; that is,
When FA and FR balance, or become equal, there is no net force; that is,
Sometimes it is more convenient to work with the potential energies
between two atoms instead of forces. Mathematically, energy (E) and
force (F) are related as:
in which EN, EA , and ER are respectively the net, attractive, and repulsive
energies for two isolated and adjacent atoms.
The bonding energy for these two atoms, E0 , corresponds to the
energy at this minimum point; it represents the energy that would be
required to separate these two atoms to an infinite separation.
Types of Bonding
Primary bonding: e- are transferred or shared Strong (100-1000
KJ/mol
or 1-10 eV/atom)
♪ Ionic Bonding:
A columbic interatomic bond that exists between two adjacent
and oppositely charged ions.
Formation of ionic bond:
1. Mutual ionization occurs by electron transfer (remember
electronegativity table)
• Ion = charged atom
• Anion = negatively charged atom
• Cation = positively charged atom
2. Ions are attracted by strong columbic interaction
• Oppositely charged atoms attract
Example: NaCl
Na has 11 electrons, 1 more than needed for a full outer shell (Neon)
Cl has 17 electrons, 1 less than needed for a full outer shell (Argon)
Note : relative sizes of ions: Na shrinks and Cl expands
The percent ionic character of a bond between elements A and B (A
being the most electronegative) may be approximated by the expression
%ionicchara cter {1 exp[ (0.25)( X A X B ) 2 ]} X 100
Where XA and XB are the electronegativities for the respective elements.
Ionic Bonds are:
♣ Very strong.
♣ Nondirectional bonds (ions may be attracted to one another in any
direction). that is, the magnitude of the bond is equal in all
directions
around an ion. It follows that for ionic materials to be
stable,
The attractive bonding forces are columbic; that is, positive and negative
ions, by virtue of their net electrical charge, attract one another. For two
isolated ions, the attractive energy EA is a function of the interatomic
distance according to:
Where:
A
1
40
( Z 1e)( Z 2 e)
An analogous equation for the repulsive energy is:
In these expressions, A, B, and n are constants whose values depend on
the particular ionic system. The value of n is approximately 8.
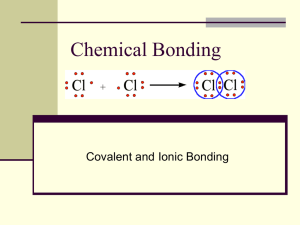
♪ Covalent Bonding:
A primary interatomic bond that is formed by the sharing of
electrons between neighboring atoms.
The simplest example is the H2 molecule, where the electrons spend
more time in between the nuclei than outside, thus producing bonding.
Figure: Schematic representation of covalent bonding in a molecule
of methane (CH4).
Formation of covalent bonds:
• Cooperative sharing of valence electrons
• Can be described by orbital overlap
• Bonds - in the direction of the greatest orbital overlap
• Covalent bond model: an atom can covalently bond with at most 8-N’,
N’ = number of valence electrons
Example: Cl2 molecule. ZCl =17 (1S2 2S2 2P6 3S2 3P5)
N’ = 7, 8 - N’ = 1 can form only one covalent bond
Covalent Bonds are:
♣ Highly directional.
♣ May be very strong, as in diamond, which is very hard and has a
very
high melting temperature, 35500C, or they may be very
weak, as with
bismuth, which melts at about 2700C).
♪ Metallic Bonding:
A primary interatomic bond involving the nondirectional
sharing
of nonlocalized valence electrons (‘‘sea of electrons’’)
that are
mutually shared by all the atoms in the metallic
solid.
Valence electrons are detached from atoms, and spread in an 'electron
sea' that "glues" the ions together.
♣ A metallic bond is non-directional (bonds form in any direction).
Figure: Schematic illustration of metallic bonding.