Mickey Mouse Molecule
advertisement
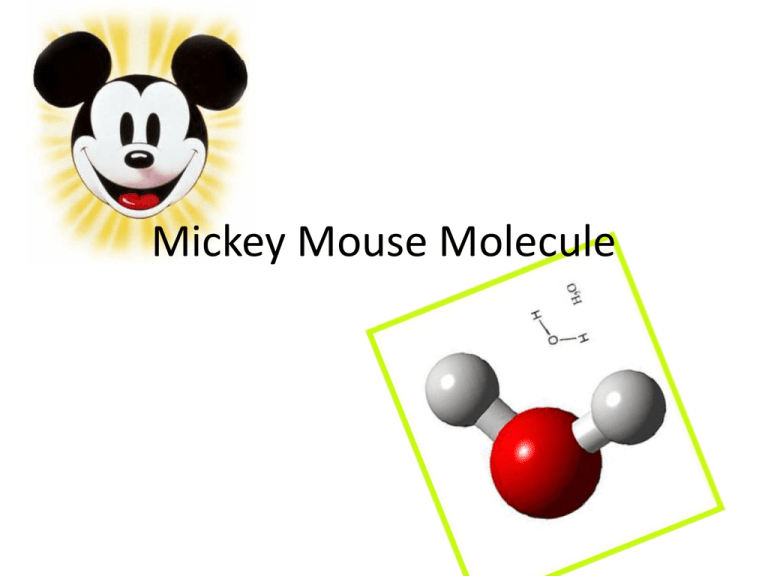
Mickey Mouse Molecule Step One • Color the pieces – the nuclei need to be the same color as their respective atoms. All atoms of the same element should be the same color. • All electrons should be colored the same color. Make it a totally different color than what you have used thus far. Step Two • Cut out the pieces and… Match the nuclei to their respective atoms. Match the electrons to their respective atoms. Call me over and get your worked checked before you proceed to gluing!!! Step Three • Glue the nuclei and electrons to their respective atoms. • DO NOT CONNECT ATOMS YET! • Locate a sheet of notebook paper. Step Four • How many electrons are in oxygen’s outer shell? • An atom needs to have its outermost electron shell FULL in order to be stable. – Is oxygen stable? BONDING • The oxygen atom is going to SHARE hydrogen’s electrons. • This is called a COVALENT BOND. • Covalent bonds are strong, but ionic bonds are stronger! Step Five • Stabilize your oxygen atom! What do you notice about this molecule? • Unequal sizes of atoms • Unequal sharing of electrons • Results in a POLAR COVALENT BOND • “ears” have _____ charge • “head” has _______charge • Label your molecule! So what? Hydrogen bonding! Hydrogen Bonding • The weakest type of bond, more of an attraction… • But incredibly powerful!!! • Bottle capped with cloth – it overcame the force of gravity! Because water molecules are sticky… • • • • ADHESION – stick to other things COHESION – stick together SURFACE TENSION STAYS A LIQUID OVER A HUGE TEMPERATURE RANGE – moderates temperature… • UNIVERSAL SOLVENT – dissolves things! • http://www.visionlearning.com/library/module_v iewer.php?mid=57 Ice = less dense than liquid water