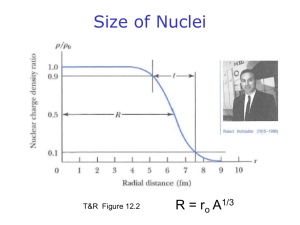
Chapter 25 Review Questions

Chapter 25 Review Questions
Atoms
Stable
Do not decay
Beta
Unstable
Alpha
Gamma Electron Capture Positron
****NOTE: The forms of radioactive decay on the right side of the diagram are not in any particular order*****
Match each numbered choice on the right with the correct radiation type on the left a. alpha b. beta c. gamma
1. high speed electron
2. 2+ charge
3. no charge
4. helium nucleus
5. blocked very easily
6. electromagnetic radiation b a c a a c
Describe the difference between a balanced nuclear equation and a balanced chemical equation .
In both, mass and matter is conserved. However, the elements that appear on the reactant side of a chemical equation are the same as those that appear on the product side, only rearranged. In a nuclear equation, the reactants and products are represented as different elements. One has transmutated into another.
Ex. Chemical
2 AlBr
3
3 BaS
3 BaBr
2
Al
2
S
3
Nuclear
36
16
S
1
0
36
15
P
Explain the difference between positron emission and electron capture
Both processes involve the conversion of a proton to a neutron. In electron capture, an electron is sucked into the nucleus and combines with a proton to produce a neutron. In positron emission, a proton decays to form a neutron and a positron.
Define Transmutation. Are all nuclear reactions also transmutation reactions?
Transmutation is the conversion of an atom of one element into an atom of another element. All nuclear reactions are transmutation reactions whether they occur naturally (decay by alpha, beta, positron, or electron capture) or whether they are induced (bombardment).
Complete the following equations.
214
83
Bi
2
4
He
210
81
Tl
239
93
Np
239
94
Pu
0
1
Half Life
The half-life of Pa-234 is 6.75 hours. How much (what fraction) of a sample remains after 20.25 hours? (1/8)
Initial=
Final= n
20 .
25 hr
6 .
75 hr
3 fraction
1
2
3
1
8 t ½ = 6.75 hours
Time elapse= 20.25 hours n= 3
The half-life of Rn-222 is 3.823 days. What was the original mass of a sample of this isotope if 0.0500g remains after 7.646 days? (0.200 g)
Initial= X
Final= 0.0500g n
7 .
646 days
3 .
823 days
2 initial
0 .
0500 g
2
1
0 .
200 g
2 t ½ = 3.823 days
Time elapse= 7.646 days n= 2
What is the half –life of a substance that decays from 192mg to 3mg in 72.4ms? (12.067ms)
Initial= 192 mg
Final= 3 mg
3
192
1
64
1
2 n n
6 t ½ =
72 .
4
6
12 .
067 ms t ½ = X
Time elapse= 72.4 ms n= 6
How long will it take for 115.2g of Ra-236 to decay to 7.2g if the half-life of this isotope is 1.4x 10
14
yrs? (5.6x10
14 yrs)
Initial= 115.2g
Final= 7.2
7 .
2
115 .
2
1
16
1
2 n n
4 TE
( 4 )( 1 .
1 x 10
14
)
5 .
6 x 101
4 t ½ = 1.4 x 10 14 yrs
Time elapse= X n= 4
Nuclear equations (Write them)
Bombardment of Cl-35 with a neutron produces S-34 and another particle
35 Cl
17
0
1 n
34
16
S
0
1
yrs
Neutron bombardment of Pu-239 gives three neutrons, La-145 and another nucleus
239
94
Cl
0
1 n
3
0
1 n
145
57
La
91
37
Rb
Tritium (H-3) and deuterium (H-2) fuse to produce 2 neutrons and another nucleus
1
3
H
1
2
H
2
0
1 n
2
4
He
Bombardment of U-238 with C12 gives an isotope of element 98 and four identical particles
238
U
92
12
6
C
250
Cf
98
4
0
0
(gamma particles)
Decay type (circle one)
As-65 Alpha, Beta or Positron/electron capture
Es-252
Re-162
Alpha , Beta or Positron/electron capture
Alpha, Beta or Positron/electron capture
Both these isotopes are stable because their neutron to proton ratio is between 1:1 and 1.5:1
Mo-85 Alpha, Beta or Positron/electron capture
Alpha decay occurs when the atomic number of the element is greater than 83
Beta decay occurs when the neutron to proton ratio is too high (greater than 1.5:1). It effectively reduces the number of neutrons and increases the number of protons.
Positron emission and electron capture occur when the neutron to proton ratio is too low
(below 1:1). They effectively increase the number of neutrons and decreases the number of protons.