Types of Nuclear Reaction
advertisement

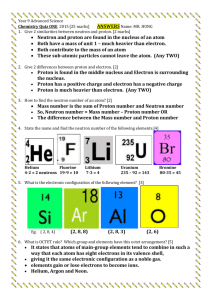
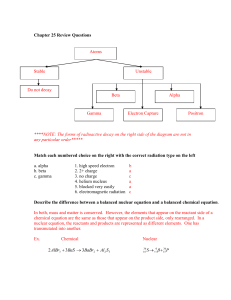
Types of Nuclear Reaction L3 1) Natural Radioactive Decay A) Alpha Decay B) Beta Decay C) Proton Emission D) Neutron Emission E) Positron Emission F) Orbital Electron Capture 2) Artificial Transmutation 3) Fission 4) Fusion All nuclear reactions are accompanied by the emission of some level of Gamma Radiation. Gamma Radiation has no charge and no mass. It is energy similar to light that can penetrate up to 1 foot of lead, or 3 feet of concrete. Natural Radioactive Decay Definition: Spontaneous release of energy and particles from the nucleus. The rate of a natural radioactive decay is measured in half-lives. Alpha Decay Definition: Release of alpha particles from the nucleus Alpha particles have a +2 charge and a mass of 4 amu. They contain 2 p+ and 2 n0 making them identical to a Helium nucleus. 4 Symbols: He or 2 Examples: 12 8 4 B Li He 5 3 2 144 140 4 Nd Ce He 60 58 2 Alpha particles can be stopped by a sheet of paper. Beta Decay Definition: The breakdown of a nucleus by the release of a Beta Particle. Beta Particles have a -1 charge and a mass of 1/1836 amu. They are a high speed electron that is coming out of the nucleus. 0 e or Symbols: 1 Beta particles are produced by the breakdown of a neutron into a beta particle and a proton. This results in an increase in the Atomic Number of the atom, but no change in its Mass Number. Examples: 11 11 0 Be B e 4 5 1 45 45 0 K Ca e 19 20 1 Beta particles can not penetrate more than a few inches into solid materials. They can be stopped by 1/4 " of aluminum. Proton Emission Definition - the breakdown of the atom by the release of a proton. This lowers both the Mass Number and Atomic Number by 1. Example: 37 20𝐶𝑎 9 5𝐵 → 36 19𝐾 + 11𝐻 → 84𝐵𝑒 + 11𝐻 Neutron Emission Definition – the breakdown of the atom by the release of a neutron. This lowers the Mass Number by one, but does not change the Atomic Number. This forms an isotope of the original atom. Example: 9 3𝐿𝑖 16 6𝐶 → 83𝐿𝑖 + 10𝑛 → 15 6𝐶 + 10𝑛 Positron Emission A positron is a particle with the same mass as an electron, but a +1 charge. It is the antimatter form of an electron. A positron is created by the splitting of a proton to form a positron and a neutron. The positron is ejected from the nucleus and the neutron stays. This reduces the Atomic Number by 1, but the Mass Number is unchanged. Example: 186 77𝐼𝑟 → 186 76𝑂𝑠 20 11𝑁𝑎 → 20 10𝑁𝑒 + + 0 +1𝑒 0 +1𝑒 Orbital Electron Capture An electron in the lowest energy level is pulled into the nucleus where it combines with a proton to form a neutron. This causes a decrease in the Atomic Number by 1, but no change in the mass of the atom. Example: 125 54𝑋𝑒 20 11𝑁𝑎 + + 0 −1𝑒 0 −1𝑒 → → 125 53𝐼 20 10𝑁𝑒 Artificial Transmutation Definition: Change in the nucleus of the atom due to its absorbing a particle provided by scientists. First produced by Rutherford (1919). Example: 238 1 239 239 0 U n U Np e 92 0 92 93 1 239 94 Pu 0 1 e Fission Definition: neutron. Splitting of a large nucleus into 2 smaller nuclei by bombarding it with a Example: 235 1 143 90 1 U n Ba Kr 3 n E 92 0 56 36 0 This process results in a small loss of mass. The mass is converted into energy (heat, light and gamma radiation). (E = mc2) If the neutrons released by the reaction strike other U-235 atoms they will split those atoms causing the reaction to continue resulting in a chain reaction. Chain reaction - any self sustaining reaction Uncontrolled Chain Reaction - If the chain reaction is allowed to continue at its own pace the reaction speed increases rapidly resulting in an explosion. This is what happens in a bomb. Controlled Chain Reaction - A material is added that can capture neutrons, limiting the number of additional atoms split. By adjusting the number of neutrons available to split other atoms the rate of the reaction can be controlled. This is what happens in a nuclear reactor. Fusion Definition - Joining of 2 small nuclei together to form a larger nucleus. Example: 2 3 4 H H He n 0 E 1 1 2 This reaction requires temperatures above 1 million degrees Celsius. These temperatures occur naturally in stars and at the center of an atom bomb explosion. The Hydrogen bomb uses a sample of Hydrogen-2 and Hydrogen - 3 at the center of a fission bomb. When the Plutonium - 239 explodes is heats the Hydrogen atoms while driving them toward each other providing the energy needed for fusion to occur. The fusion reaction results in a mass loss. The mass is converted to energy. Since the mass loss in fusion is a higher percentage of the total mass present, the fusion reaction gives off more energy than a fission reaction. Stars due to their higher temperatures and pressures can utilize Hydrogen-1 in their fusion reactions. Research is underway to develop a way to run a controlled fusion reaction that we can use to generate electricity.