P301_2009_week13
advertisement

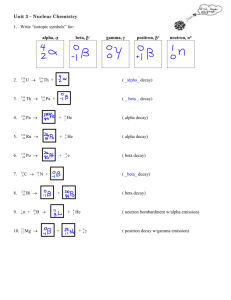
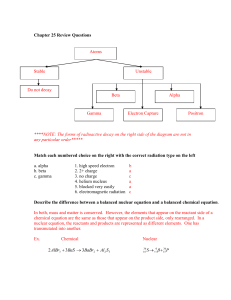
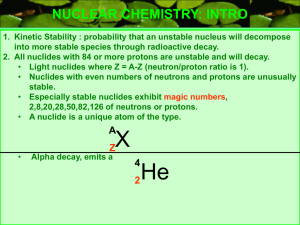
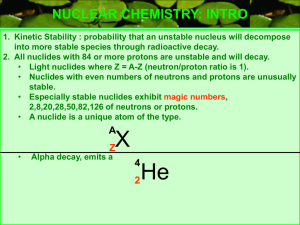
Size of Nuclei T&R Figure 12.2 R = ro A1/3 Shell model for Nuclei From E. Segre “Nuclei and Particles” 3-D Harmonic Oscillator Spherical Square well Trends in Nuclear Stability T&R Figure 12.5 See also Nudat2 at: http://www.nndc.bnl.gov/nudat2/ Trends in Nuclear Stability T&R Fig. 12.6 S-Process http://en.wikipedia.org/wiki/S-process Types of Radiation •Alpha (a): •4He nucleus; very easy to stop (paper, dead skin etc.) •Beta (b) •Electrons or positions, relatively easy to stop •Gamma (g) •High-energy photons (of nuclear origin) •X-rays •High-energy photons (of atomic origin) •Auger Electrons •Neutron (n) •Ion Types of Radiation http://www.nndc.bnl.gov/nudat2/reColor.jsp?newColor=dm Alpha Decay T&R Fig. 12.11 http://en.wikipedia.org/wiki/File:Alpha1spec.png Lecture 23 Potential Barrier: Alpha decay The deeper the “bound” state is below the top of the barrier, the lower will be the kinetic energy of the alpha particle once it gets out, and the slower will be the rate of tunneling (and hence the longer the half-life). Figures from Rohlf “Modern Physics from a to Zo”. Beta Radiation Decay http://education.jlab.org/glossary/betadecay.html Beta Radiation DecayNeutrinos The energy of the b particle (electron or positron) is not fixed, this led Pauli to suggest that another unobserved (unobservable?) particle must also be involved in the decay. The “neutrino” was the name given by Fermi a few years later after he developed a theory for the above curve (and after Chadwick discovered the neutron). About 10 of the CALM respondents last night did not quote this as the reason for the energy distribution in beta decay). Typical Decay scheme Most alpha, beta, EC, n, fission etc. decay (but not all, 210Po for example) leave the daughter nucleus in an excited state, and a gamma ray is (eventually) produced to take the daughter to its ground state. http://en.wikipedia.org/wiki/Gamma_ray Typical Decay scheme II Nuclei can decrease their proton number by one in three ways, positron emission (the most common) Electron capture (much more rarely; see next slide), or proton emission (very rare). http://www.nucleide.org/DDEP_WG/Nuclides/Na-22_tables.pdf Electron Capture An alternative to positron emission (in which a proton converts to a neutron within the nucleus by emitting a positively charged particle) is “electron capture” in which an atomic electron is absorbed by the nucleus (also converting the proton to a neutron). This event will most likely take place when the energy available in the decay is less than that needed to create a positron. •What kind of electron would most likely be involved? • What signatures might you expect from such an event? •Examples: 7Be, 37Ar, 41Ca, 49V, 51Cr, 53Mn, 57Co, 58Ni http://www.euronuclear.org/info/encyclopedia/e/electroncapture.htm