Chapter 2 Describing Motion
advertisement

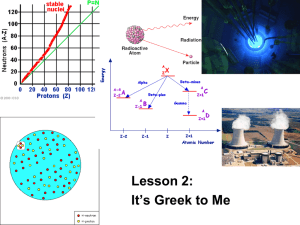
Nuclear Reactions Elementary Particles The only atomic particles that play a part in nuclear reactions are the protons and the neutrons; electrons do not play a part in nuclear reactions. We use the following notation to describe the specific element we are considering in a nuclear reaction. X represents the element, Z represents the atomic number (number of protons), and A represents the atomic mass number (the number of protons and neutrons) of the element. A Z X Isotopes For the nuclides below, determine the number of neutrons and protons. A # p #n Z #p 87 41 Nb 48 24 Cr 60 29 Cu 44 18 Ar 147 69 Tm Atomic Radii Use the formula below in order to find the atomic radii of the following Nuclide. r 1.2 10 m A 15 28 12 Mg 1 3 Particle Masses Nuclear masses are specified in unified atomic mass units or amu The atomic mass of a neutron is 1.008665 u. The atomic mass of a proton is 1.007276 u. A neutral hydrogen atom 11H , which has an electron and a proton but no neutron, has a mass of 1.007825 u. We will use the mass of a neutral hydrogen in the place of the mass of a proton. The mass of a 24 He nucleus is known to be 4.002602 u. Compare this mass to the masses of the appropriate number of protons and neutrons combined. 2m0 p 2m 1n 2(1.007825u) 2(1.008665u) 1 0 What did you discover? 4.03298u In the question above, you found that the actual mass was less than the mass of its constituent parts. What do you think happened to the “missing mass?” Binding Energy The difference in masses discovered on the previous slide is known as the total binding energy of the nucleus. This energy represents the amount of energy that must be put into the nucleus in order to break apart its protons and neutrons. For a given nucleon Az X, the total binding energy, Eb, is Eb 931.5MeV Zm 1H ( A Z )mn m A X 1 Z Find the amount of energy put into the following nuclide in order to break it apart. 24 12 Mg Nuclides This notation is very important because it allows us to represent different isotopes of an element X. For a given atom, carbon for instance, nuclei are found that contain different numbers of neutrons even though they contain the same amount of protons. Here are the symbols of some different isotopes of carbon. They are all Carbon because they all have 6 protons; however, they have different numbers of neutrons. These “different” carbons are known as Isotopes of each other. The carbon to the left is known as “Carbon 12” because it has six neutrons and six protons. The carbon to the right is known as “Carbon 16” and has six protons and ten neutrons. A Z X 12 6 C 13 6 C 14 6 C 15 6 C 16 6 C Alpha Decay Alpha decay is one of several types of nuclear decay. In alpha decay a parent nucleus is broken apart to yield a daughter nucleus and an alpha particle. The general equation for alpha decay is as follows. An alpha particle is a neutral helium atom. A Z X A4 Z 2 Y He 4 2 Q-Value In alpha decay, the masses of the daughter nucleus and alpha particle combined are less than the mass of the parent nucleus. The “missing mass” is converted into the kinetic energy of the alpha particle and the daughter nucleus. This “missing mass” or released energy is known as the disintegration energy. It is also known as the Q-value for the particular parent nucleus. The equation used to determine the Q-value is Q m A X m A 4Y m 4 He c 2 Z 2 Z 2 Find the daughter nuclide due to alpha decay and the Q-values or the nuclides below. 209 83 Bi Beta Minus Decay In Beta decay a parent nucleus is broken apart to yield a daughter nucleus and a Beta particle. A A Beta particle is either an electron (e-) or a positron (e+). Z If an electron (also known as a -) is emitted during the decay process, then the decay process is known as a “Beta minus decay.” The general equation for beta minus decay is as follows. The underlying reaction is the conversion of a neutron into a proton, electron, and an anti-neutrino. A Z X A Z 1 Y X Z A1Y Neutron Proton n p Beta Minus Decay •Calculate the KE for Beta Minus decay for A Z X Y A Z 1 32 15 32 15 P n p 32 P 16 S e m M p (M s e ) 0 KEmax M mass of the nucleus m (M p 15e ) (M s e 15e ) m (M p 15e ) (M s 16e ) m mp ms Q=[mAZ - mAZ+1 ]c2 Beta Plus Decay If a Positron (also known as a +) is emitted during the decay process, then the decay process is known as a “Beta plus decay.” The general equation for beta plus decay is as follows. The underlying reaction is the conversion of a proton into a neutron, positron, and a neutrino. A Z X A Z 1 Y Neutron Proton A Z X Z A1Y p n Beta Plus Decay •Calculate the KE for Beta Minus decay for A Z X Y A Z 1 22 11 22 11 Na n p 22 Na 10 Ne e m M Na (M Ne e ) 0 KEmax M mass of the nucleus m (M Na 11e ) (M Ne 11e e ) m (M Na 11e ) (M Ne 10e 2e ) m mNa mNe 2e Q=[mAZ - mAZ-1 -2me ]c2 Electron Capture In electron capture, an orbital electron is captured by the nucleus, combines with a proton, and forms a neutron and a neutrino. The general equation for electron capture is as follows. The underlying equation for electron capture is the conversion of a proton and an electron into a neutron and a neutrino. A Z X A Z 1 Y A Z Neutron Proton X Y A Z 1 p n