Answers
advertisement

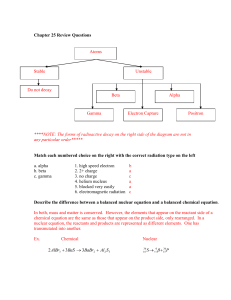
Day 8.3: Radioactivity and Conservation Laws (11.3, 13.1) Radioactivity was completely unexpected and discovered over a hundred years ago. Nuclei of one element could turn into nuclei of another element and emit three different particles; alpha, beta or gamma. 1) When passed through a magnetic field pointing into the page, the alpha turns left, the beta turns right and the gamma is not deflected. What does that tell you about the particles? The alpha are positive, the beta negative and the gamma neutral. 2) The beta turned much more than the alpha. What does that tell you? The beta has less A) mass B) speed C) velocity D) momentum The radius of the curve for the beta is smaller. This means it must have a smaller mass and/or speed or a larger charge. F = ma means qvB = mv2/r and so r = mv/qB = P/qB. It must have a smaller momentum or a larger mass. 3) The speeds of the , and particles were 0.05 c, 0.8 c and c. Describe how a velocity selector can be used to measure the speeds of two of these particles. In a velocity selector, the electric force is balanced the magnetic force (qvB = qE) which means the speed of a particle going straight through is given by v = E/B. The gamma particle is neutral so it won’t experience an electric or magnetic force. 4) It turns out that these were not new particles. What were they? What are their masses and charges? Alpha – helium nucleus, charge 2e, mass 4u, made of 2 protons and 2 neutrons. Beta – electron, charge 1e, mass 0.00055 u (about 1/2000 that of the proton) Gamma – high energy photon, no charge and no mass Alpha decay: Parent -> Daughter + He + energy 5) Radioactive decay conserves the number of nucleons (protons plus neutrons) and charge. Po-218 undergoes alpha decay. What does it produce? A) Po – 214 B) Po-216 C) Pb-214 D) Pb-216 It loses 2 charges and 4mass units. Pb-214 6) Mass-energy is conserved via E = mc2. How much energy is released when U (m = 236.045562 u) decays by emitting an alpha particle (m = 4.002602 u) to form Thorium (m = 232.038051 u)? (Note: u = 1.66054 x 10-27 kg = 1.4923 x 10-10 J/c2 = 931.5 MeV/c2.) What happens to this energy? m=(236.045562-4.002602-232.038051)u = 0.004909u = 0.004909x931.5 MeV/c2=4.573 MeV/c2 It goes into the kinetic energy of the two particles and possibly a gamma photon if the daughter nucleus was left in an excited state. 7) Momentum is conserved. a) Draw a diagram of the momenta of the two products. Draw a diagram of the two velocities. b) Calculate the ratio of the velocities. Calculate the ratio of their kinetic energies. Mv = mV. The ratio of the velocities is the inverse of the ratio of the masses 232 /4.00 = 58.0 E = ½ mv2 = ½ p/m. The ratio of the kinetic energies will also be 58.0. The alpha particle carries away the bulk of the energy. Beta Decay: Parent -> Daughter + e + energy 8) Ba-141 undergoes beta decay. What does it produce? A) La-141 B) La-145 C) Cs-141 D) Cs-145 The nucleus has one neutron eject an electron and turn into a proton, so the atomic number goes up by one. There is no change to the atomic number. La-141 9) Mass-energy is conserved via E = mc2. How much energy is released when Na-24 (m = 23.990961 u) decays by emitting an electron (m = 0.000548579867u) to form Mg-24 (m = 23.985042 u)? m=(23.990961-0.000548579867-23.985042)u=0.005370421u~ 0.005370x931.5MeV/c2=5.003MeV/c2 10) Momentum is conserved. a) Draw a diagram of the momenta of the two products. Draw a diagram of the velocities. b) Calculate the ratio of the velocities. Calculate the ratio of the kinetic energies. 23.985/0.00054858 = 43,722. (This can’t be drawn to scale in the diagram above.) c) The electron does get most of the energy, but not the calculated ratio. The emitted electrons have a range of energies and momenta, from almost zero up to a maximum. The neutrino was proposed in 1930 to account for this. (It was detected in 1956, about 25 years later.) Explain how having a third particle will result in a range of momenta for the electron. Include a diagram for minimum and for maximum electron energy. If the neutrino is ejected opposite the nucleus, the electron will have no momentum. If the neutrino is ejected in the same direction as the nucleus, the electron will have maximum. p. 673 #1, p. 676 # 1 - 8, p. 736 # 2, 4, 5, p. 740 #20, p. 743 #37-39