DAY 1: ATOMIC STRUCTURE & AVERAGE ATOMIC MASS
advertisement
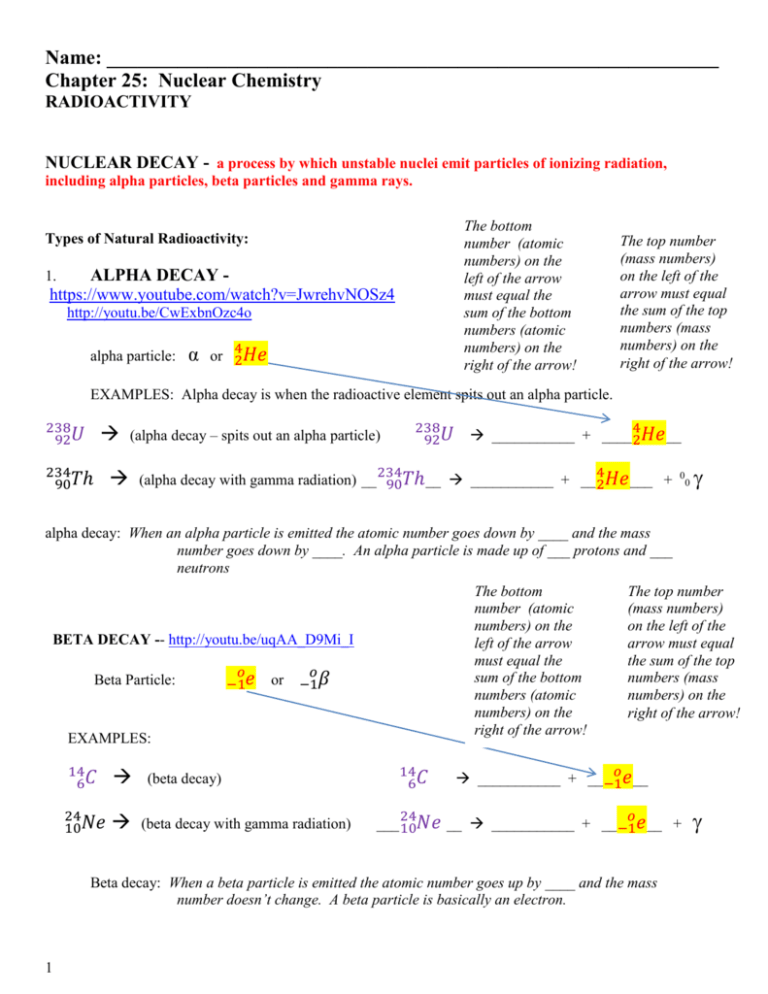
Name: _____________________________________________________________ Chapter 25: Nuclear Chemistry RADIOACTIVITY NUCLEAR DECAY - a process by which unstable nuclei emit particles of ionizing radiation, including alpha particles, beta particles and gamma rays. The bottom number (atomic numbers) on the left of the arrow must equal the sum of the bottom numbers (atomic numbers) on the right of the arrow! Types of Natural Radioactivity: ALPHA DECAY https://www.youtube.com/watch?v=JwrehvNOSz4 1. http://youtu.be/CwExbnOzc4o alpha particle: α 4 or 2𝐻𝑒 The top number (mass numbers) on the left of the arrow must equal the sum of the top numbers (mass numbers) on the right of the arrow! EXAMPLES: Alpha decay is when the radioactive element spits out an alpha particle. 238 92𝑈 234 90𝑇ℎ (alpha decay – spits out an alpha particle) 238 92𝑈 4 ___________ + ____ 2𝐻𝑒 __ 234 4 (alpha decay with gamma radiation) __ 90𝑇ℎ__ ___________ + __ 2𝐻𝑒 ___ + 0 0 alpha decay: When an alpha particle is emitted the atomic number goes down by ____ and the mass number goes down by ____. An alpha particle is made up of ___ protons and ___ neutrons The bottom number (atomic numbers) on the left of the arrow must equal the sum of the bottom numbers (atomic numbers) on the right of the arrow! BETA DECAY -- http://youtu.be/uqAA_D9Mi_I Beta Particle: 𝑜 −1𝑒 or 𝑜 −1𝛽 EXAMPLES: 14 6𝐶 24 10𝑁𝑒 (beta decay) (beta decay with gamma radiation) 14 6𝐶 The top number (mass numbers) on the left of the arrow must equal the sum of the top numbers (mass numbers) on the right of the arrow! 𝑜 ___________ + __ −1𝑒 __ 24 𝑜 ___ 10𝑁𝑒 __ ___________ + __ −1𝑒 __ + Beta decay: When a beta particle is emitted the atomic number goes up by ____ and the mass number doesn’t change. A beta particle is basically an electron. 1 3. What is gamma radiation? And, why is it so harmful? because gamma radiation has high ____________ and high ______________________ so it is very penetrating to the cells of living things. Comparing Alpha, Beta and Gamma Radiation Alpha radiation has a positive charge (because of the two protons). It is the least penetrating and therefore the least dangerous. Beta radiation has a negative charge. It is “in the middle” in terms of penetrating power. Gamma radiation is energy. It has no mass. It is the most harmful since it is the most penetrating of all three natural emissions. PRACTICE: 60 27𝐶𝑜 2 Write equations for the following nuclear reactions: (alpha decay with gamma radiation ) _________ ___________ + __________ + 131 53𝐼 (beta decay ) 40 19𝐾 (positron emission ) __________ ___________ + __________ __________ ___________ + ____________ 109 46𝑃𝑑 (alpha decay ) __________ ___________ + __________ + 269 110𝐷𝑠 (beta decay with gamma radiation) __________ ___________ + __________ + PRACTICE: Nuclear Reactions 0 1. 42 19𝐾 −1𝑒 + ___________________ 4 2. 239 94𝑃𝑢 2𝐻𝑒 + __________________ 231 3. 235 92𝑈 ________________ + 90𝑇ℎ 0 4. __________________ 40 20𝐶𝑎 + −1𝛽 5. 147𝑁 + 10𝑛 ______________ + 11𝐻 0 6. _________________ + 10𝑛 239 93𝑁𝑝 + −1𝛽 1 7. 113 48𝐶𝑑 + 0𝑛 __________________ + 8. 21𝐻 + _______________ 42𝐻𝑒 + 10𝑛 4 1 9. 239 92𝑈 + 2𝐻𝑒 _______________ + 0𝑛 10. 11𝐻 + 31𝐻 ___________________ 11. 49𝐵𝑒 + 11𝐻 _______________ + 42𝛼 12. 63𝐿𝑖 + 10𝑛 42𝐻𝑒 + _________________ 54 2 13. 56 26𝐹𝑒 + 1𝐻 _____________ + 25𝑀𝑛 54 1 14. 209 83𝐵𝑖 + 24𝐶𝑟 0𝑛 + ____________________ 3 Half-Life - http://youtu.be/WAsmY4ocWSA half life: PROBLEMS: Use the example in number 1 to solve the rest. 1. The half life of Potassium-40 is 1.3billion years. How much of a 4.00g sample of Potassium-40 remains after 5.2billion years? Cut the mass in half for each half life that passes 4.00g 2.00g 1.00g 0.5g 0.25g Total time = # half life cycles Half life () 5.2 billion = 4 1.3 billion 2. The half life of francium is 22 minutes. How much of a 2000g sample of francium remains after 66 minutes? Hint: how many half-lives have happened? 3. Find the half-life of an isotope if 50.0g of the isotope decays to 6.25g in 3600 years. Hint: how many half-lives have happened Cut the mass in half for each half life that passes 3600 years = 1200 years is the half life 3 50.0g 25.0g 12.5g 6.25g (so 3 half lives passed) 4. A sample of francium originally has a mass of 40.0g. When the scientist returns from lunch, the mass of francium in the sample has decreased to 5.0g. If the half-life of francium is 22 minutes, how long was the scientist’s lunch break? Hint: how many half-lives have happened 4 Practice Half-Life 1. How much of a 100.0g sample of 198Au is left after 8.10 days if its half life is 2.70 days? 2. A 50.0g sample of 16N decays to 12.5g in 14.4 seconds. What is its half life? 3. What is the half life of 99Tc if a 500g sample decays to 62.5g in 639,000 years? 4. The half life of 232Th is 1.4 x 1010 years. If there are 25.0g of the sample left after 2.8 x 1010 years, how many grams were in the original sample? 5. The half life of 42K is 12.4 hours. How much of a 750g sample is left after 62.0 hours? 5 OTHER NUCLEAR REACTIONS: SKIP this page FOR NOW 1. POSITRON EMISSION: http://youtu.be/bjuZSvZukAw 𝑜 +1𝑒 EXAMPLES: 8 3𝐵 (positron emission ) __________ ___________ + __________ 15 8𝑂 (positron emission) __________ ___________ + __________ 2. BETA CAPTURE (also known as electron capture): http://youtu.be/sg_XoUDsP08 . Beta Particle: 𝑜 −1𝑒 or 𝑜 −1𝛽 EXAMPLES: 6 204 84𝑃𝑜 (beta capture with x rays ) 188 76𝑂𝑠 (beta capture with x rays ) __________ + ___________ __________ + x-rays __________ + ___________ __________ + x-rays TRANSMUTATION REACTIONS: FUSION: joining together of nuclei; produces an uncontrollable amount of energy STEP 1: 𝟑 + 𝟏𝑯 𝟐 𝟏𝑯 𝟓 𝟐𝑯𝒆 STEP 2: 𝟓 𝟐𝑯𝒆 𝟒 𝟐𝑯𝒆 + 𝟏 𝟎𝒏 + energy (SUNLIGHT) FISSION: splitting of nuclei STEP 1: 𝟏 𝟎𝒏 + 𝟐𝟑𝟓 𝟗𝟐𝑼 𝟐𝟑𝟔 𝟗𝟐𝑼 STEP 2: 𝟐𝟑𝟔 𝟗𝟐𝑼 7 𝟏𝟒𝟒 𝟓𝟔𝑩𝒂 + 3 𝟏𝟎𝒏 + 𝟖𝟗 𝟑𝟔𝑲𝒓 + energy 8








