PY3004 Atomic and Molecular Spectroscopy
advertisement

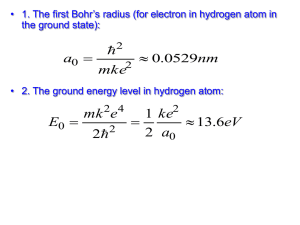
JS Atomic and Molecular Spectroscopy Tutorial 1 1. The figure below shows the spectrum of an A0 dwarf star, which has an effective surface temperature of about 10,000 K. The spectrum contains a number of absorption lines from the Balmer series of the hydrogen atom. a. Using the Bohr model, identify four lines in the spectrum, starting with the line at the longest wavelength. b. Draw an energy level diagram for hydrogen, showing the transitions involved in producing the four lines identified in (a). Note: RH = 1.0967757 x 107 m-1 2. Write down the mathematical form of the radial wavefunction (Rnl ( r ) ) for an electron in the 1s orbital. a. Calculate the corresponding radial probability function (P( r )). b. Sketch the radial wavefunction and its probability function. Describe how they behave when r 0 and when r . c. Prove that the maximum probability of finding the electron occurs at r = a0 for the hydrogen atom. (Hint: dP( r )/dr = 0 at maximum). 3. The positronium atom consists of a positron and an electron orbiting about their common centre of mass. a. Show that the wavelengths of the emitted lines are twice that of hydrogen. b. What is the estimated distance between the electron and the positron? c. The positronium atom disintegrates rapidly, producing two photons. What is the energy and resulting wavelength of these photons? d. Identify the electron-positron annihilation line the spectrum below. Observed spectrum Theoretical models