4: 7, 18, 22, 30, 35
advertisement
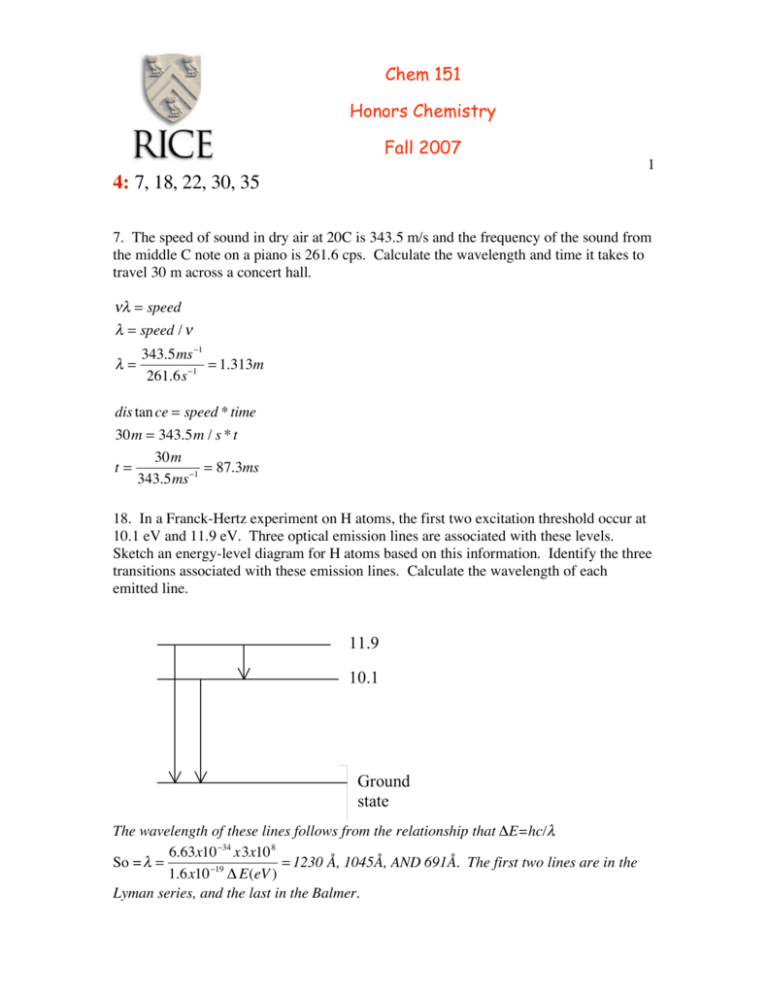
Chem 151 Honors Chemistry Fall 2007 1 4: 7, 18, 22, 30, 35 7. The speed of sound in dry air at 20C is 343.5 m/s and the frequency of the sound from the middle C note on a piano is 261.6 cps. Calculate the wavelength and time it takes to travel 30 m across a concert hall. !" = speed " = speed / ! "= 343.5ms #1 = 1.313m 261.6s #1 dis tan ce = speed * time 30m = 343.5m / s * t 30m t= = 87.3ms 343.5ms !1 18. In a Franck-Hertz experiment on H atoms, the first two excitation threshold occur at 10.1 eV and 11.9 eV. Three optical emission lines are associated with these levels. Sketch an energy-level diagram for H atoms based on this information. Identify the three transitions associated with these emission lines. Calculate the wavelength of each emitted line. The wavelength of these lines follows from the relationship that ∆E=hc/λ 6.63x10 "34 x3x10 8 = 1230 Å, 1045Å, AND 691Å. The first two lines are in the So = ! = 1.6x10 "19 ! E(eV ) Lyman series, and the last in the Balmer. 2 22. The Be3+ ion has a single electron. Calculate the frequencies and wavelengths of light in the ion’s emission spectrum for the first three lines in each of the series that are analogous to the Lyman and Balmer series of neutral hydrogen. In what region of the spectrum does this radiation lie? Since there is only one electron, we can use the Bohr equations, but must now realize that Z=4. Thus eq 15.11 becomes # 1 # 1 1& 1& ! = 3.29x1015 *16 % 2 " 2 ( = 5.26x1016 % 2 " 2 ( $ n f ni ' $ n f ni ' For the Lyman lines, nf=1, and ni=2,3,4 (for the first three lines). For the Balmer series, nf=2, (see fig 15.19) and ni=3,4,5. Thus for the Lyman lines, the frequencies are (3.9450 4.6756 4.9313)x1016 giving wavelengths of (7.65, 6.41 & 6.08) nm. For the Balmer, the frequencies are ( 0.7306 0.9862 1.1046)x1016. The wavelengths are 41.06, 30.4 & 27.2 nm. These are wavelengths that lie in the X-ray region of the spectrum. 30. Suppose we picture an electron in a chemical bond as being a wave with fixed ends. Take the length of the bond to be 1Å (0.1nm) a) Calculate the wavelength of the electron wave in its ground state and in its first excited state. The groundstate has wavelength 2Å and the first excited state 1Å b) How many nodes does the excited state have? One. (we won’t count the ends because all of the modes have fixed ends.) (see fig) 35. a) The position of an electron is known to be within 10Å. What is the minimum uncertainty in the velocity? ∆p∆x>h/4π ∆x≈10Å (10x10-10m), so ∆p>6.63x10-34Js/(4πx10-9m)= 5.27x10-24Js/m=∆(mv) m=9.1x10-31kg, so ∆v=5.27x10-24/9.1x10-31 = 5.8x104m/s b) Repeat this calculation for a helium atom. ∆p>6.63x10-34Js/(4πx10-9m)= 5.27x10-24Js/m=∆(mv) m=4x1.66x10-27kg, so ∆v=5.27x10-24/4x1.66x10-27= 7.9m/s









