Exam 2 Review
advertisement
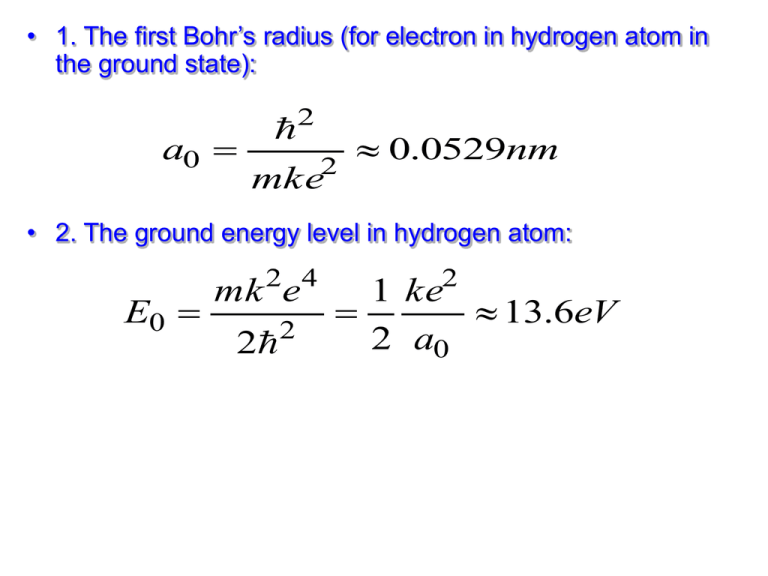
• 1. The first Bohr’s radius (for electron in hydrogen atom in the ground state): a0 2 2 0.0529nm mke • 2. The ground energy level in hydrogen atom: E0 mk 2e 4 2 2 1 ke2 13.6eV 2 a0 3. The energy levels, orbit radius, kinetic energy, linear, and angular momentum of electron in an atom: E0 En Z 2 n 1 2 kZe2 K E mv 2 2r 2 me ke e 2 p me v r 4. The Bragg condition: 2d sin n , L mvr n 1,2,3,4..... 5. Useful Formulas for Wavelength and Energy: E hf hc f c 6. The lines wavelength in atomic spectrum: 1 1 R 2 2 n m 1 n>m 7. The wavelength for a transition from state n to state (n-1): hc E n E n 1 8. The uncertainty principle: xp Et 2 h 2 9. The normalization condition: 2 dx 1 0 10. The de Broglie relation for energy, frequency, and wavelength of the particle: E hf hc h p c hc f Ei E f 11. Standing wave condition for a particle in a box of width L: Ln n 2 p2 K 2m p h 15. The probability of finding the particle in some region b from a to b: P a 2 ( x) dx 16. The wave function and the energy levels for a particle in the box of length L: n ( x) 2 nx sin L L En n2h 2 8mL2 1. A monochromatic beam of light is absorbed by a collection of ground-state hydrogen atoms in such a way that six different wavelengths are observed when the hydrogen relaxes back to the ground state. (a) What is the wavelength of the incident beam? (b) What is the longest wavelength in the emission spectrum of these atoms? (c) To what portion of the electromagnetic spectrum does it belong? (d) To what series does it belong? (e) What is the shortest wavelength? (f) To what series does it belong? 2. (a) How much energy is required to cause an electron in hydrogen to move from the n = 1 state to the n = 2 state? (b) Suppose the electron gains this energy through collisions among hydrogen atoms at a high temperature. At what temperature would the average kinetic energy 3kBT/2, where kB is the Boltzmann constant, be great enough to excite the electron? 3. Suppose the ionization energy of an atom is 4.10eV. In the spectrum of this same atom, we observe emission lines with wavelengths 310nm, 400 nm, and 1377.8 nm. Use this information to construct the energy level diagram with the fewest levels. Assume that the higher levels are closer together. Sketch this energy level diagram. 4. The positron is the antiparticle to the electron. It has the same mass and a positive electric charge of the same magnitude as that of the electron. Positronium is a hydrogenlike atom consisting of a positron and an electron revolving around each other. Using the Bohr model, find the allowed distances between the two particles and the allowed energies of the system.











