Chemistry 199 - Oregon State University
advertisement

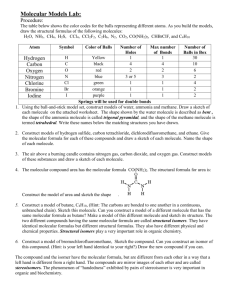
Chemistry 201/211 Worksheet 1 Fall 2004 September 30, 2004 Oregon State University 1. Sketch a cartoon of the PTE (Periodic Table of the Elements). Identify the metal and non-metal regions with labels and a "staircase." 2. List six metals by name and symbol. Examples: lithium Li, lead Pb 3. List six non-metals by name and symbol. Examples: carbon C, argon Ar 4. NaCl is known as "table salt" or sodium chloride. What group number is sodium in? What group number is chlorine in? Is NaF expected to exist? Explain. Is LiF expected to exist? Explain. Is MgF expected to exist? Explain. Sodium (Na) is in group 1. Chlorine (Cl) is in group 17. NaF and LiF are expected to exist since they are electrically neutral ionic compounds. MgF is not expected to exist since it is not electrically neutral. Mg has a 2+ charge (group 2) and F has a 1charge (group 17) Note: Chlorine (Cl) is in group 17. The chloride ion (Cl-) is in group 18. Sodium (Na) is in group 1. The sodium ion (Na+) is in group 18. 5. CaCl2, calcium chloride, has many applications (http://www.dow.com/calcium/) including water treatment. What group number is calcium in? Is CaF2 expected to exist? Explain. Is LiCl2 expected to exist? Explain. Is MgF2 expected to exist? Explain. Calcium is in group 2, chloride is in group 18. Calcium fluoride (CaF2) is an electrically neutral ionic compound. Ca is in group 2. Lithium chloride (LiCl2) is not expected to exist since Li is 1+ and Cl is 1- giving the compound a total charge of 1-. MgF2 is electrically neutral and is expected to exist. 6. Lithium oxide, with the formula Li2O, readily absorbs carbon dioxide and water vapor from the air. What group number is lithium in? What group number is oxygen in? Is Li2F expected to exist? Explain. Is Na2S expected to exist? Explain. Is Cs2Se expected to exist? Explain. Lithium is in group 1. Oxygen is in group 16. Li2F is not expected to exist because it is not electrically neutral (1+); Na2S and Cs2Se are expected to exist because they are electrically neutral. 7. Magnesium selenide, with the formula MgSe, is a light brown powder that is unstable in air. What group number is magnesium in? What group number is selenium in? Is MgO expected to exist? Explain. Is CaS expected to exist? Explain. Is BaCl expected to exist? Explain. Magnesium (Mg) is in group 2. Selenium (Se) is in group 16. MgO and CaS are electrically neutral and are expected to exist. Ba has a 2+ charge, Cl has a 1- charge so BaCl would have a 1+ charge, and therefore it is not electrically neutral and is not expected to exist. 8. What is the formula of sodium oxide? Sodium (Na) is a group 1 metal and is 1+. Oxide is the monatomic (one atom) anion of oxygen and has a 2- charge. For electric neutrality the formula is Na2O. 9. Does neon form chemical compounds? Explain. Not normally. Neon is a “noble gas” and is considered chemically inert. 10. Which of the following formulas are correct (X) or incorrect? Explain why. (A) LiCl2 (F) MgCl2 X (K) AlCl2 (B) Na2O X (G) Mg2O (L) Al2O3 X (C) FrF X (H) SrI2 X (M) BeF2 X (D) Li2S X (I) CaO X (N) KS (E) K2S X (J) BaCl (O) KNe 11. Which of the following sets of elements will form a molecular compound? Explain. (a) Ni and Cu. No, both are metals (b) Li and F. No, the resulting compound would be ionic (c) Li and Mg. No, both are metals (d) C and Cl. Yes, both are nonmetals (e) He and Mg. No, He does not react (yet?) 12. List a pair of elements that will form a molecular compound. S and O are examples. Most nonmetals would work. 13. Which of the following substances is an ionic compound? Explain. (a) Octane. (C8H18) No, it is made of only nonmetals. (molecular compound) (b) Carbon dioxide. (CO2) No, it is made of only nonmetals. (c) Sodium fluoride. (NaF) Yes, a metal and a nonmetal combining (d) Dinitrogen pentoxide. (N2O5) No, it is made of only nonmetals. (e) Acetic acid. (HC2H3O2) No, it is made of only nonmetals. 14. List a pair of elements that will form an ionic compound. Any group 1 or 2 element combined with a nonmetal from group 14-17 15. How many bonds does carbon form? How many bonds does nitrogen form? How many bonds does oxygen form? How many bonds does fluorine form? How many bonds does argon form? Carbon – 4; Nitrogen-3; Oxygen - 2; Fluorine – 1; Argon - 0 16. Sketch a molecule that contains two carbon atoms and six hydrogen atoms. Name this molecule. What is the molecular formula of this molecule? What is the condensed structural formula of this molecule? Ethane; C2H6; CH3 CH3 17. Sketch a molecule that contains two nitrogen atoms and four hydrogen atoms. What is the molecular formula of this molecule? What is the condensed structural formula of this molecule? The name of this molecule is hydrazine. Hydrazine is a colorless oily liquid that fumes in air. N2H4; NH2NH2 18. Sketch a molecule that contains a carbon atom, three chlorine atoms, and a fluorine atom. 19. Sketch a molecule that contains only two nitrogen atoms. Sketch a molecule that contains only two oxygen atoms. Sketch a molecule that contains only two chlorine atoms. 20. Sketch pentane. What is the molecular formula of pentane? What is the condensed structural formula of pentane? C5H12; CH3CH2CH2CH2CH3 21. Sketch octane. What is the molecular formula of octane? What is the condensed structural formula of octane? C8H18; CH3CH2CH2CH2CH2CH2CH2CH3 22. What is the name of MgCl2? What is the name of PCl3? What is the name of NO? What is the name of N2O4? What is the name of SF6? What is the name of AlF3? What is the name of CH3CH2CH2CH2CH2CH3? What is the name of CH3CH3? magnesium chloride phosphorus trichloride nitrogen monoxide dinitrogen tetroxide (or tetraoxide) sulfur hexafluoride aluminum fluoride hexane ethane