LibrMolProjChem211F`..
advertisement

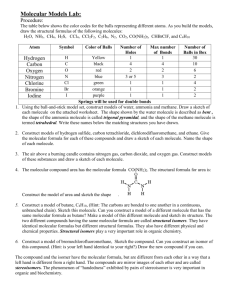
Molecule Library Project Chem 211 Name 1. Compound Names My molecule Fall 2008 References: Molecular Formula CAS Registry Number [see Lehman Appendix VII] CA Index Name Other names. What type of name is it? (IUPAC, systematic, common, generic or trade) IUPAC or systematic Name Common, Generic or Trade 2. Physical Properties References: o (at normal laboratory conditions, 20-25 C and 1 atmosphere) Boiling point Melting point Refractive index Color Density Under normal lab conditions, would your compound be a solid, liquid or gas? Why do you say so? Chem 211 Molecule Library Project Fall 2008 3. Structure References: (draw by hand, a skeletal representation of your molecule using wedges and dashes as needed to specify unique structure) 4. Molecular Model Make a molecular model of your compound. If your model kit doesn’t have enough atoms, use the model kits provided so you can make a complete structure. Hold your model in your hands. Manipulate it. What do you learn about your molecule from the model? You might comment on shape, distribution of heteroatoms, flexibility, etc. (~100 words) Look at and manipulate the model of a classmate’s molecule. In what ways are the molecules alike? How are they different? (~50 words) Only write about what you learn by considering the molecular model. Comparison molecule belongs to (classmate’s name) Alike: Different: 2 Chem 211 Molecule Library Project Fall 2008 3 Chem 211 Molecule Library Project Fall 2008 5. Functional groups References: Draw your molecule using molecular drawing software or paste in the structure from an online source. Then indicate functional groups present by circling (or highlighting) them on the structure and by drawing arrows to them and labeling them. Functional groups you should consider are those in CGWW Table at the bottom of p. 36 and if you have nitrogen in a ring, see CGWW pp. 1180-1181. 6. Aqueous Solubility References: Would you predict that your compound is soluble in water? Why or why not? Consider the number and distribution of any polar heteroatoms present. Might your compound be more soluble in aqueous sodium hydroxide or aqueous hydrochloric acid than it is in water? (see Chem 211 Lab Manual Appendix B). Why or why not? 4 Chem 211 Molecule Library Project Fall 2008 7. Stereochemical Features References: Draw the structure of your molecule (hand drawn, created using molecular drawing software, or pasted in from an online source) in which stereochemical features are clearly indicated, for example using wedges and dashes. Circle (or highlight) and draw an arrow to label any cis-trans structures or chiral centers. Label any chiral centers correctly as R or S (See http://www.acdlabs.com/iupac/nomenclature/93/r93_630.htm and http://www.chemhelper.com/enantiomers.html for explanations of how to determine the configuration of a chiral center. If your molecule has no cis-trans features or any chiral centers, write none and explain how you came to that conclusion. Then label the cis-trans structures and chiral centers of the following structure. H O H Cl 8. Infrared Spectrum References: Draw the structure of your molecule (hand drawn, created using molecular drawing software, or pasted in from an online source). Circle the bonds with distinctive IR absorbance. List the characteristic IR frequencies expected or reported for your compound. Be as precise as possible with “cm-1” (for ex. an ester C=O absorbs at different cm-1 than a ketone C=O) Bond or structural feature Bond or structural feature , cm-1 , cm-1 5 Chem 211 Molecule Library Project Fall 2008 6 Chem 211 Molecule Library Project Fall 2008 9. Essay: Write a short essay about your compound (300-400 words) at a level that would interest other young scientists; consider your audience to be a senior chemistry or biology major. You might address these questions or anything else that intrigues you: What is it? How was it discovered? Isolated? Identified? Why is it interesting to scientists? What sort of research is being carried out on this compound? Among your references you must include a primary source (mark with *), secondary source, (mark with #), and tertiary source (mark with ^). You must provide documentation in your report folder of the primary source you used (either the first page or the entire research article if you copied it all). 7 Chem 211 Molecule Library Project Fall 2008 10. Reference List 8