211-11MolLibProj
advertisement

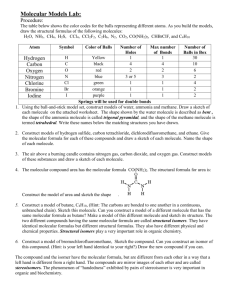
CHEMISTRY 211 Fall 2011 Molecule Library Project Goals: This project is designed to give you a chance to apply your developing understanding of organic molecules to learn about an interesting substance and acquire a feeling for the diversity of structures and properties among organic compounds. Another objective is for you to learn how to use Chemical Abstracts, which you will access on-line through SciFinder Scholar software, as well as other reference materials commonly used by chemists. Completion of this project is required to pass CHEM 211 and your completed project will account for 3 % of your course grade in the course. Library Orientation: In class in on Wednesday, September 14, we will explore the tools available through Reeves Library and on the Internet that allow you to complete this project. The Report: The report is organized into the nine sections enumerated below. More information on each section is provided in the attached report form. Report Outline 1. Compound Names 2. Physical Properties 3. Structure 4. Molecular Model 5. Functional groups 6. Aqueous Solubility 7. Infrared Spectrum 8. Essay 9. References Use the attached paper copy of the report form (pp. 5-10) or the electronic version (MolRpt.doc on the course website) to collect information as you do your research. Use an electronic version of the report form for your report; it must be word-processed (Times or Times New Roman, 12 point with 1 in. margins: top, bottom and both sides). In your report, do NOT change the order of the questions or their page placement; that is, do not change the page breaks provided in the file. If you require more space than is available on a given page, allow it to spill over to a new page, but leave the next page break in the original position. Your report will be submitted in two phases. Phase one will include sections 1->6 with cited references in section 9 and a copy of the original information you gathered. Phase two will consist of a corrected version of sections 1->6, sections 7and 8, and an expanded reference list in section 9. On the dates specified below for phase one and phase two, turn in your report with all records related to your literature search attached with a binder clip. Your records should include any printouts you made of source materials, your hand written or electronic “working” report form, and any other notes documenting your search path. The pages of the report are to be stapled together, but the attached record sheets should not be stapled. There are specific requirements for citing the references that you use to find the required information about your molecule. Include all of your references in a numerical list in section 9 at the end of the report. You do not have to cite the suggested CGWW readings. For sections 1-7 of your report, cite specific references for each section by including their numbers from the section 9 reference list in the space designated for references at the beginning of each section. (On the right side of the first line of each section) For your section 8 essay, place citation numbers as superscripts at the end of the sentence in which you report information from a particular reference. For example a citation for information from reference 3 (See list below) would appear as: Even though acetaminophen is considered an effective and safe over-the-counter analgesic, it is the preferred method of suicide for adolescents in Great Britain.3 This research paper examines other safety concerns related to this commonly used medication. (See citation for 3 in the Sample Section 9 reference List on p.2) Fall 2011 2 Molecule Library Project All references used to prepare your report are listed in Section 9. These references are numbered and listed in the order they are cited in your report. The sample Reference List below shows the required format for citing books, periodicals and Internet pages. If you are unable to find required information about your molecule, list all the references you searched in the answer space on the report form. Sample Section 9 Reference List 1. Physician's Desk Reference, 58th edition, Thomson PDR, 2004. 2. The Merck Index, 11th edition, Susan Budavari, ed., Merck & Co., 1989. 3. “Acute acetaminophen overdose in adolescents and adults.”, Hamm J., Critical Care Nurse, 2000, 20, 69-74. 4. “The effect of analgesic agents on the healing rat medial collateral ligament.”, Hanson C. A., Am J Sports Med, 2005, Feb. 16. (abstract only). 5. MedScape DrugInfo: ACETAMINOPHEN ORAL. http://www.medscape.com/druginfo/Pharm?id=11866&name=ACETAMINOPHEN+ORAL&DrugType=1&MenuID=PHM&ClassID=N&Ge neralStatement=N(accessed October 13, 2011). Explanation of Reference Formats above: # 1 shows the format for citing a book with an author: book title, edition (both in italics), author, publisher, year of publication. # 2 shows the format for citing a book with an editor: book title, edition (both in italics), editor name (followed by ed.), publisher, year of publication. # 3 shows the format for a periodical reference: “article title” (in quotation marks), authors, periodical title, publication year, volume, inclusive pages. (all in italics) # 4 shows a periodical reference in the format of #3 above, but only the abstract of the article was read. # 5 shows the format for citing an Internet reference: Author (if any). Title of Site. URL (accessed date). Note: When citing information found using online resources, provide the information for the actual source (web page or journal), NOT the search engine or database URL.] Classification of References from University of Maryland Libraries http://www.lib.umd.edu/guides/primary-sources.html Accessed 8/18/11 Primary Sources: original materials. They have not been filtered through interpretation or evaluation. Primary sources are original materials based on research. They are usually the first formal appearance of results in print or electronic format. They present original thinking, report a discovery, or share new information. E.g. #’s 3 & 4 above Secondary Sources: less easily defined than primary sources. They are interpretations and evaluations of primary sources. Secondary sources are not evidence, but rather commentary on and discussion of evidence. Examples include encyclopedias, review articles, handbooks, bibliographies, and abstracts/indexes. E.g. #’s 1, 2 & 5 above Tertiary Sources: consist of information that is a distillation and collection of primary and secondary sources such as textbooks. Comparative Examples: SUBJECT PRIMARY Chemistry/Life Sciences Einstein's diary SECONDARY Book on Einstein's life TERTIARY Dictionary on Theory of Relativity Molecule Library Project 3 Fall 2011 Dates and Deadlines 9/8 Thurs. Sign up for molecule outside 213 Collier. Only 1 person per 9/9 Fri. molecule and one molecule per person. You are required to sign up by Friday, September 9 at 4:00 PM. Make sure that you record the complete correct name of your molecule 9/21 Wed. 8:50 AM Library orientation in class: Be sure to bring the assignment and report form to class. 10/31 Mon. 4:00PM Phase one of report due: complete sections 1 – 6 w/ references in 9. 12/5 Mon. 4:00PM Phase two of report due: turn in the original copy of your phase 1 report and a revised copy of your phase 1 report (1-6) with sections 7, 8 and expanded references, section 9. Compounds abscisic acid lidocaine abyssinone II linalool adapalene α-linoleic acid alizarin luteolin amentoflavone lutein astaxanthin metformin 1,2-benzenedithiol octocrylene benzoic anhydride octyl methoxycinnamate bisphenol a oseltamivir bupivacaine 2,6-nonadienal butylated hydroxytoluene pantothenic acid carbon disulfide phenolphthalein celecoxib propranolol cerivastatin rosiglitazone cetirizine sanguinarine cholecalciferol sulfanilamide complanadine A sulfolane curcumin tafamidis 3,4-diaminopyridine 2,3,7,8-tetrachlorodibenzo-p-dioxin docetaxel Δ9-tetrahydrocannabinol epicatechin thiourea eriochrome black T triptycene fexofenadine urushiol ganoderic acid warfarin gemifloxacin whiskey lactone gossyplure p-xylene z-guggulsterone yohimbine indigo yuccagenin (+)-kavain zingerone levoglucosenone zymosterol Report Form (next 6 pages) Chem 211 Molecule Library Project Name 1. Compound Characteristics Name of my molecule Fall 2011 References from section 9: Molecular Formula CAS Registry Number CA Index Name Other names. What type of name is it? (IUPAC or systematic names– define the structure of the compound; common, generic or trade names – do not provide structural information) Name 2. Physical Properties (at 1 atmosphere) Boiling point Refractive index IUPAC or systematic Common, Generic or Trade References from section 9: Melting point Color Density Based only on the physical properties listed in this section, do you expect your compound to be a solid, liquid or gas under normal lab conditions (20-25oC and 1 atmosphere)? Provide a warrant to support your claim. Fall 2011 2 Molecule Library Project 3. Structure References from section 9: (draw by hand, a bond-line representation of your molecule) 4. Molecular Model Make a molecular model of your compound. If your model kit doesn’t have enough atoms, use the model kits provided in the chemistry reading room (2** Collier) so you can make a complete structure. Hold your model in your hands. Manipulate it. What do you learn about your molecule from the model? You might comment on shape, distribution of heteroatoms, flexibility, etc. (~100 words) Look at and manipulate the model of a classmate’s molecule. In what ways are the molecules alike? How are they different? (~50 words) Only write about what you learn by considering the molecular model. Comparison molecule name Alike: Different: belongs to (classmate’s name) Chem 211 3 Fall 2011 5. Functional groups References from section 9: Draw your molecule using molecular drawing software or paste in the structure from an online source. Then indicate functional groups present by circling (or highlighting) them on the structure and labeling them. Functional groups you should consider are those in CGWW Table at the bottom of p. 36 and if you have nitrogen in a ring, see CGWW pp. 1180-1181 for ring names. 6. Aqueous Solubility References from section 9: (Consult Appendix A1 of the lab manual and your results from Experiment 5 for guidelines on solubility in water, acid and base.) Would you predict that your compound is soluble in water? Consider the number and distribution of any polar heteroatoms present and provide a warrant to support your claim. Might your compound be more soluble in aqueous sodium hydroxide or aqueous hydrochloric acid than it is in water? Provide a warrant to support your claim. 3 Fall 2011 4 Molecule Library Project 7. Infrared Spectrum References from Section 9: Draw the structure of your molecule (hand drawn, create it using molecular drawing software, or pasted it in from an online source). Circle the bonds with distinctive IR absorbance. List the characteristic IR frequencies expected or reported for your compound. Be as precise as possible with “cm-1” (for ex. an ester C=O absorbs at different cm-1 than a ketone C=O) (See 211 Lab Manual Appendix C) Bond or structural feature , cm-1 Bond or structural feature , cm-1 Chem 211 5 Fall 2011 8. Essay: Write a short essay about your compound (300-400 words) at a level that would interest other young scientists; consider your audience to be a senior chemistry, biology, neuroscience or environmental studies major. You might address the following questions or anything else that intrigues you: What is it? How was it discovered? Isolated? Identified? Why is it interesting to scientists? What is it used for? What sort of research is being carried out on this compound? Among your references you must include at least one primary source (mark with *). You must provide documentation of the primary source(s) you used in the records related to your literature search (either the first page with the citation or the entire research article if you copied it all). 5 Fall 2011 9. Reference List 6 Molecule Library Project