Organic Chemistry Molecular Models Assignment Use the molecular
advertisement

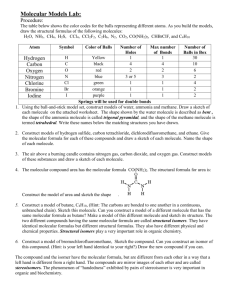
Organic Chemistry Molecular Models Assignment Use the molecular model kit to build and name all alkanes and their isomers up to six carbons. On your own paper, write the structural formulas and names of each molecule. Use the molecular model kit to build and name all alkenes and their isomers up to six carbons. Include only the positional isomers (location of the double bond) and not any branched isomers. On your own paper, write the structural formulas and names of each molecule. Use the molecular model kit to build a model of benzene (C6H6). J-mol software for molecular modeling TYPE THE FOLLOWING ADDRESS INTO YOUR BROWSER: http://introchem.chem.okstate.edu/DCICLA/jml/jmol.php 1. Choose a molecule off of the drop-down menu 2. Click and drag to rotate (or choose the “spin” box) 3. To find bond angles, double click on a peripheral atom, single click on a central atom, and then drag to another peripheral atom 4. Select the bond dipoles box to see polar covalent bonds within the molecule 5. Select the molecular dipole box to see the overall polarity (dipole moment) of the molecule 6. State the name of the molecule when the molecular formula is given. Write the molecular formula of the molecule when the name is given MOLECULES TO EXAMINE: CH4 C2H2Cl2 (1,1; cis-1,2; trans-1,2) CH3CH3 methylamine CH2CH2 methanol CHCH methylfluoride (what is this molecule’s IUPAC name) C6H6 (make sure multiple bond box is checked) 1-butene trans-2-butene cis-2-butene H2CO methylpropene