Stereochemistry Summary
advertisement

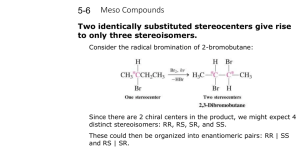
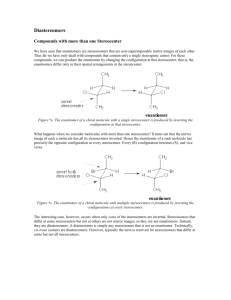
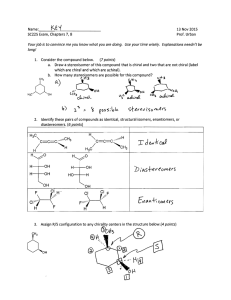
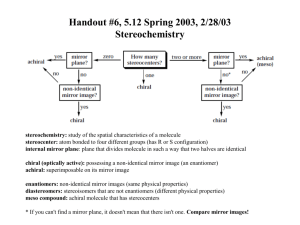
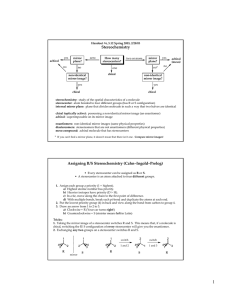
5 Important Concepts 1. Isomers – Same molecular formula, different compounds: • • • Constitutional – Individual atoms are connected differently Stereoisomers – Same connectivity, different 3D arrangement. Mirror-Image Stereoisomers – Related as image 2. Chiral Molecule – Not superimposable on its mirror image 3. Stereocenter – Carbon atom bearing 4 different substituents 4. Enantiomers – Two stereoisomers, each a nonsuperimposable mirror image of the other Important Concepts 5 5. Racemate – A one-to-one mixture of enantiomers 6. Mirror Plane – Chiral molecules cannot contain a mirror plane. 7. Diastereomers – Stereoisomers not related to each other as mirror images (i.e. cis/trans). 8. Two Stereocenters In A Molecule – Create up 9. to 4 stereoisomers: 2 diastereomerically related pairs. If the 2 stereocenters generate a mirror plane in the molecule, the molecule is known as a meso compound and is achiral. Physical Properties of Stereoisomers – Most are the same except for the rotation of plane-polarized light. One enantiometer rotates the plane of polarization to the right, the other to the left. This rotation is expressed as the specific rotation []. 5 Important Concepts 10. Absolute Configuration – Determined by X-ray diffraction. Assignment of R or S, as determined by the Cahn, Ingold, and Prelog sequence rules 11. Fischer Projections – Standard 2-D representation of 3-D molecules containing stereocenters 12. Radical Halogenation – Can introduce chirality into an achiral compound. Racemic mixtures are often produced. 13. Radical Halogenation of a Chiral Molecule Containing 1 Stereocenter – • • Gives a racemate if the reaction occurs at the stereocenter Gives 2 diastereomers in unequal amounts if the reaction occurs elsewhere 5 Important Concepts 14. Stereoselectivity – Preference for the formation of one stereoisomer when several are possible 15. Resolution – Separation of enantiomers • Reaction with a pure enantiomer of a second chiral compound and separation of the diastereomers • Chiral chromatography