Diastereomers Sparknotes
advertisement

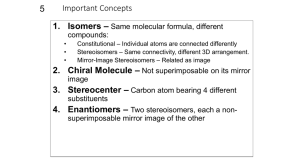
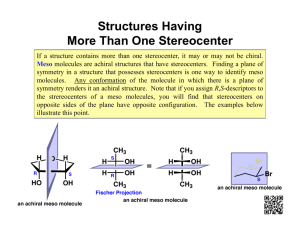
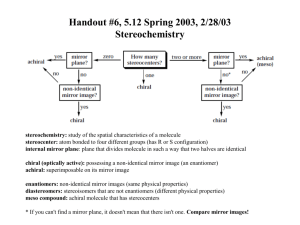
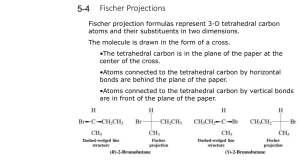
Diastereomers Compounds with more than one Stereocenter We have seen that enantiomers are stereoisomers that are non-superimposable mirror images of each other. Thus far we have only dealt with compounds that contain only a single stereogenic center. For these compounds, we can produce the enantiomer by changing the configuration at that stereocenter; that is, the enantiomers differ only in their spatial arrangements at the stereocenter. Figure %: The enantiomer of a chiral molecule with a single stereocenter is produced by inverting the configuration at that stereocenter. What happens when we consider molecules with more than one stereocenter? It turns out that the mirror image of such a molecule has all its stereocenters inverted. Hence the enantiomer of a such molecule has precisely the opposite configuration at every stereocenter. Every (R) configuration becomes (S), and vice versa. Figure %: The enantiomer of a chiral molecule with multiple stereocenters is produced by inverting the configurations at every stereocenter. The interesting case, however, occurs when only some of the stereocenters are inverted. Stereoisomers that differ at some stereocenters but not at others are not mirror images, so they are not enantiomers. Instead, they are diastereomers. A diastereomer is simply any stereoisomer that is not an enantiomer. Technically, cis-trans isomers are diastereomers. However, typically the term is reserved for stereoisomers that differ at some but not all stereocenters. Figure %: Diastereomers formed by inverting some but not all stereocenters. The following example should help clarify any lingering confusion about the stereochemical jargon that has been presented thus far in this chapter. Consider 2-bromo-3-chlorobutane, which has stereocenters at C 2 and C 3 . In general, a molecule with n stereocenters has 2^n stereoisomers, so there are a total of four possibilities for 2-bromo-3-chlorobutane: Figure %: Four possible stereoisomers of 2-bromo-3-chlorobutane Each of the four stereoisomers of 2-bromo-3-chlorobutane is chiral. There are two pairs of enantiomers. Any given molecule has its enantiomer; the two other molecules are its diastereomers. Summary of Isomerism The following flowchart summarizes the relationships between different types of isomerism: Figure %: Summary of Isomerism Meso Compounds A compound that contains exactly one stereocenter is always chiral. But compounds that contain more than one stereocenter are not necessarily chiral. This occurs when multiple stereocenters create an internal plane of symmetry. For example, consider 2,3-dichlorobutane. There are four possible stereochemical permutations since there are two stereocenters: Figure %: Stereoisomers of 2,3-dichlorobutane Note, however, that compounds 3 and 4 are not enantiomers. Rather, they are identical (flip 3 over to obtain 4). The reason for this is that the molecule has an internal plane of symmetry that bisects the 4-carbon chain. Therefore, it is achiral and is identical to its mirror image. A compound that contains multiple stereocenters but is achiral due to an internal mirror plane is called a meso compound. So there are really only three stereoisomers of 2,3-dichlorobutane: a pair of chiral molecules (enantiomers of each other) and the achiral meso compound. 1 SparkNotes Editors. “SparkNote on Organic Chemistry: Enantiomers and Diastereomers.” SparkNotes LLC. n.d.. http://www.sparknotes.com/chemistry/organic3/enantiomersanddiastereomers/ (accessed March 14, 2013).