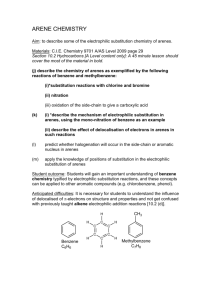
Ortho, Para-Directing Groups
advertisement

EAS Rxns of Substituted Benzenes • Substituents on a benzene ring can affect two things: 1) Location of subsequent substitution rxns 2) Reactivity of ring toward further substitutions 1 Directing Effects of Substituents • Ortho-para directing group: substituent that directs substitution rxns to the ortho and para positions on a benzene ring – Tend to be alkyl groups or groups with lone pairs on atoms directly bonded to ring 2 Ortho, Para-Directing Groups • Rxn of an electrophile at the para position of anisole • Rxn of an electrophile at the meta position of anisole – The charge on the meta-derived intermediate cannot be delocalized onto the -OCH3 group 3 Ortho, Para-Directing Groups 16.5 Electrophilic Aromatic Substitution Reactions of Substituted Benzenes 4 Ortho, Para-Directing Groups • Alkyl substituted benzene rings have a similar explanation 16.5 Electrophilic Aromatic Substitution Reactions of Substituted Benzenes 5 The Ortho, Para Ratio • Ratio of ortho to para products = 2:1? • Specific ratios are sometimes due to spatial demands, but many cases are less easily explained • Fortunately, ortho and para products often have different physical properties and can be separated 16.5 Electrophilic Aromatic Substitution Reactions of Substituted Benzenes 6 • Meta directing group: substituent that directs substitution rxns to the meta positions on a benzene ring – Polar groups – Positive or partial positive charges next to ring – Do not have lone pair on atom directly bonded to ring 7 Meta-Directing Groups 8 9 Problems • Predict the major products for the following rxns: 1) Nitration of chlorobenzene 1) Bromination of nitrobenzene 10 Activating and Deactivating Effects • Activating group: A group that causes the substituted benzene ring to react more rapidly than benzene itself – All ortho-para directing groups except Halogens • Deactivating group: A group that causes the substituted benzene ring to react more slowly than benzene itself – All meta directing groups 16.5 Electrophilic Aromatic Substitution Reactions of Substituted Benzenes 11 Activating and Deactivating Effects • Controlled by two simultaneously operating properties of substituents: 1) Inductive/Polar effect • Withdrawl or donation of electrons through a σ bond due to electronegativity and polarity of bonds in a functional group 12 13 2) Resonance effect • Ability of substituent to stabilize carbocation intermediate in an EAS rxn through delocalization of π electrons • Withdrawal or donation of electrons through a π bond due to the overlap of a p orbital on the benzene ring and a p orbital on a substituent. • Electron withdrawing by resonance: 14 • Electron donation by resonance • Inductive and resonance effects can work in the same or opposite directions 15 Activating and Deactivating Effects 16.5 Electrophilic Aromatic Substitution Reactions of Substituted Benzenes 16 Orbital overlap may also affect the degree to which the resonance effect operates • Methoxy (-OCH3) group: – Inductive affect = weak withdrawing/decativating – Resonance affect = strongly donating/activating • Chloro group: – Inductive affect = strongly withdrawing/decativating – Resonance affect = weakly donating/activating 17 Summary of Substituent Effects 18 Problems 1) Explain why Freidel-Crafts alkylation rxns often give polysubstituted products while FC acylation rxns do not. 2) Draw resonance structures for the intermediates from the reaction of an electrophile at the ortho, meta, and para positions of benzaldehyde. Which intermediates are most stable? Least stable? 19 Use of EAS in Organic Synthesis • With two or more substituents, the activating and directing effects are roughly the sum of the effects of the individual substituents 16.5 Electrophilic Aromatic Substitution Reactions of Substituted Benzenes 20 Use of EAS in Organic Synthesis 16.5 Electrophilic Aromatic Substitution Reactions of Substituted Benzenes 21 Use of EAS in Organic Synthesis • Some EAS reactions can be carried out under very mild conditions and without a catalyst 16.5 Electrophilic Aromatic Substitution Reactions of Substituted Benzenes 22 Use of EAS in Organic Synthesis 16.5 Electrophilic Aromatic Substitution Reactions of Substituted Benzenes 23 Problems 1) At which positions do you expect electrophilic substitution to occur in the following substances? 24 2) Show the major product(s) for the rxn of the following molecules with CH3CH2Cl/AlCl3 and HNO3/H2SO4 25 Hydrogenation • Aromatic rings are resistant to hydrogenation • More extreme conditions are generally required (higher T and/or P) 16.6 Hydrogenation of Benzene Derivatives 26 Hydrogenation • The reaction cannot be selectively stopped 16.6 Hydrogenation of Benzene Derivatives 27