Chapter 18 Reactions of aromatics
advertisement

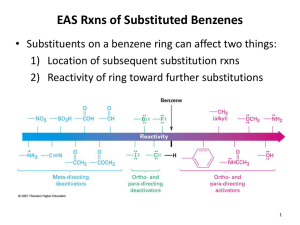
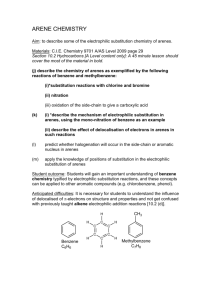
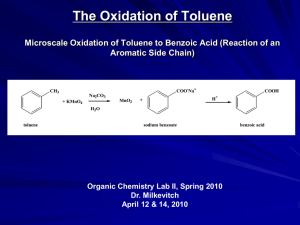
Chapter 18 Reactions of Aromatic molecules •Benzene is aromatic: a cyclic conjugated compound with 6 electrons •Reactions of benzene lead to the retention of the aromatic core Types of Reactions • Electrophilic substitution • Diazonium chemistry • Nucleophilic substitution • Misc. modification of substituents Electrophilic Substitution Reactions of Benzene and Its Derivatives Electrophilic Substitution versus addition Arenium ion: Wheland intermediate Also called sigma complex Energy diagram comparison of electrophilic substitution versus electrophilic addition Is this kinetic or thermodynamic control? Electrophilic Bromination of Aromatic Rings toluene para-bromotoluene ortho-bromotoluene meta-bromonitrobenzene Regiochemistry (ortho, para, meta) will depend on relative stability of resonance structures for carbocation intermediates Mechanism for Electrophilic Bromination of Aromatic Rings para carbocation ortho carbocation ortho carbocation Electrophilic substitution of Bromobenzene Halogens are ortho para directors, but are still deactivating compared with benzene Transformations of Aryl bromides Aromatic Nitration • The combination of nitric acid and sulfuric acid produces NO2+ (nitronium ion) • The reaction with benzene produces nitrobenzene Gun solvent Nitration of toluene: an electron rich monomer ortho & para Regioselectivity explained Kinetics are more favorable for ortho and para isomers with electron donating substitutent on benzene Too corrosive for artillery shells •High explosive: Shock wave •Secondary explosive: requires primer (primary explosive) to detonate •Nitroaromatics are harmful: aplastic anemia and liver toxicity Reactions of nitroaromatics • Nitro is deactivating (destabilizes the Wheland intermediate & slows reactions). • Reactions are 100,000 slower than with benzene • Meta directing More polynitration mono-bromination Reduction to anilines and azo compounds Nucleophilic aromatic substitution Meta selective: electron withdrawing groups •electron withdrawing group inductively destabilizes adjacent carbocations in ortho and para cases. • No adjacent carbocation in meta regiochemistry resonance structures Reduction of Nitroaromatics to Aryl amines Amine group is electron donating: changes subsequent regiochemistry from meta to ortho-para Aromatic Sulfonation • • • • Substitution of H by SO3 (sulfonation) Reaction with a mixture of sulfuric acid and SO3 Reactive species is sulfur trioxide or its conjugate acid Reaction occurs via Wheland intermediate and is reversible Alkali Fusion of Aromatic Sulfonic Acids • Sulfonic acids are useful as intermediates • Heating with NaOH at 300 ºC followed by neutralization with acid replaces the SO3H group with an OH • Example is the synthesis of p-cresol Alkylation of Aromatic Rings: The Friedel–Crafts Reaction • Aromatic substitution of a R+ for H • Aluminum chloride promotes the formation of the carbocation • Rearrangement of carbocation can occur Friedel Craft alkylation mechanism Friedel Craft alkylation with rearrangements Limitations of the Friedel-Crafts Alkylation • Only alkyl halides can be used (F, Cl, I, Br) • Aryl halides and vinylic halides do not react (their carbocations are too hard to form) • Will not work with rings containing an amino group substituent or a strongly electron-withdrawing group Control Problems • Multiple alkylations can occur because the first alkylation is activating Acylation of Aromatic Rings • Reaction of an acid chloride (RCOCl) and an aromatic ring in the presence of AlCl3 introduces acyl group, COR – Benzene with acetyl chloride yields acetophenone Friedel Craft Acylation Mechanism Acylation – No rearrangement of acylium ions Ortho- and Para-Directing Deactivators: Halogens • Electron-withdrawing inductive effect outweighs weaker electron-donating resonance effect • Resonance effect is only at the ortho and para positions, stabilizing carbocation intermediate Strongly Activating o,p directors: Multiple additions Reduce reactivity with acetyl groups Summary of activation versus deactivation and ortho & para versus meta direction With multiple substitutents there can be antagonistic or reinforcing effects Antagonistic or Non-Cooperative Reinforcing or Cooperative D = Electron Donating Group (ortho/para-directing) W = Electron Withdrawing Group (meta-directing) Multiple Substituents in antagonistic systems The most strongly activating substituent will determine the position of the next substitution. May have mixtures. O C H3 O C H3 S O 3H SO 3 O 2N H2S O 4 O C H3 + O 2N O 2N S O 3H => Sulfonic acid blocking groups ? ? Electrophilic substitution reaction used to make plastics Acid or base catalyzed reactions Acid catalyzed reaction of phenol with formaldehyde Bakelite, Novolac Electrophilic substitution reaction used to make plastics Acid catalyzed reaction of phenol with formaldehyde Electrophilic substitution reaction used to make plastics Base catalyzed reaction of phenol with formaldehyde Nucleophilic Aromatic Substitution • While protons attached to electron-rich aromatics can be displaced by electrophiles, halogens attached to electrondepleted aromatics can be displaced by nucleophies • Nucleophilic aromatic substitution is similar to SN1/SN2 in its outcome, but follows completely different mechanisms Substitution Nucleophilic Aromatic (SNAr), the Benzyne Mech, and reaction of diazonium salts SNAr Mechanism: Electron EWG’s Addition-Elimination Reactions • Typical for aryl halides with nitro groups in ortho or para position • Addition to form intermediates (Meisenheimer complexes) by electronwithdrawal • Halide ion is then lost by elimination Chlorobenzene is unreactive under ordinary laboratory conditions… …but brute force will do the job! However, this can’t be the same mechanism Nucleophilic substitution aromatic Strong EWG ortho or para to leaving group (F, Cl, Br, I, OTf) Strong nucleophile (HO-, RO-, Amide, thiolate, carbanions) SNAr used in making polymers (plastics) Elimination-Addition: Benzynes • The reaction involves an elimination reaction that gives a triple bond, followed by rapid addition of water • The intermediate is called benzyne Evidence for Benzyne as an Intermediate • Bromobenzene with 14C only at C1 gives substitution product with label scrambled between C1 and C2 • Reaction proceeds through a symmetrical intermediate in which C1 and C2 are equivalent— must be benzyne Structure of Benzyne • Benzyne is a highly distorted alkyne • The triple bond uses sp2-hybridized carbons, not the usual sp • The triple bond has one bond formed by p–p overlap and by weak sp2–sp2 overlap Another Nucleophilic Like reaction using Diazonium Salts (made from Anilines) ArNH2 is easily made from reduction of nitrobenzenes. Permits preparation of molecules impossible to make otherwise Mechanism for formation of Diazonium Salts from Anilines Three possible reaction mechanisms for Nucleophilic substitution reactions with Diazonium salts Thermal ionization (like SN1) e.g. Formation of phenol Redox (radical intermediate) Organometallic e.g. Sandmeyer reaction Diazonium chemistry 62 16.10 Oxidation of Aromatic Compounds • Alkyl side chains can be oxidized to CO2H by strong reagents such as KMnO4 and Na2Cr2O7 if they have a C-H next to the ring • Converts an alkylbenzene into a benzoic acid, ArR ArCO2H Bromination of Alkylbenzene Side Chains • Reaction of an alkylbenzene with N-bromo-succinimide (NBS) and benzoyl peroxide (radical initiator) introduces Br into the side chain Mechanism of NBS (Radical) Reaction • Abstraction of a benzylic hydrogen atom generates an intermediate benzylic radical • Reacts with Br2 to yield product • Br· radical cycles back into reaction to carry chain • Br2 produced from reaction of HBr with NBS 16.11 Reduction of Aromatic Compounds • Aromatic rings are inert to catalytic hydrogenation under conditions that reduce alkene double bonds • Can selectively reduce an alkene double bond in the presence of an aromatic ring • Reduction of an aromatic ring requires more powerful reducing conditions (high pressure or rhodium catalysts) Summary of reactions not involving aromatic substitution • Reduction of nitrobenzenes to anilines • Conversion of benzenesulfonic acids to phenols • Side chain oxidation of alkylbenzenes to benzoic acids • Side chain halogenation of alkylbenzenes • Reduction of benzenes to cyclohexanes • Reduction of aryl ketones to alkyl benzenes