Reactions of Benzene
advertisement
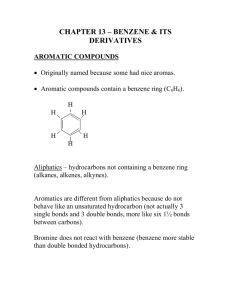
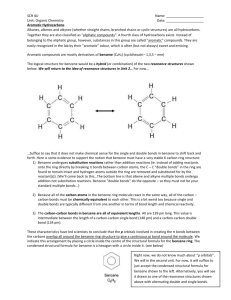
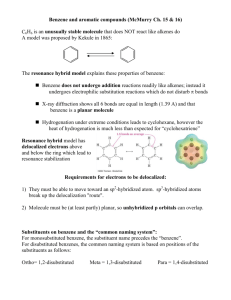
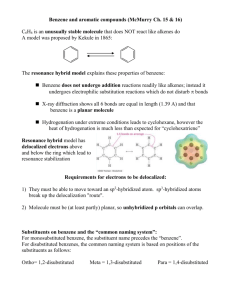
Chapter 8 Aromaticity Reactions of Benzene and Substituted Benzenes 1 The first structure for benzene was proposed by August Kekulé in 1872. 2 3 We often represent benzene as a hybrid of two equivalent structures 4 Naming Benzene Compounds To name a benzene ring with one substituent, name the substituent and add the word benzene. 5 Common Names OMe anisole styrene CH3 OH toluene phenol CHO COOH benzaldehyde benzoic acid NH2 aniline CN benzonitrile 6 ortho-dibromobenzene or o-dibromobenzene or 1,2-dibromobenzene meta-dibromobenzene or m-dibromobenzene or 1,3-dibromobenzene para-dibromobenzene or p-dibromobenzene or 1,4-dibromobenzene 7 • If the two groups on the benzene ring are different, alphabetize the names of the substituents preceding the word benzene. • If one substituent is part of a common root, name the molecule as a derivative of that monosubstituted benzene. 8 For three or more substituents on a benzene ring: 1. Number to give the lowest possible numbers around the ring. 2. Alphabetize the substituent names. 3. When substituents are part of common roots, name the molecule as a derivative of that monosubstituted benzene. The substituent that comprises the common root is located at C1. 9 10 Name the following benzene compounds. 11 Page 214 Problem 14 More practice….. Page 226 Problem 26 12 More naming….. 13 Interesting Aromatic Compounds 14 15 Reactions of Benzene: Oxidation 16 What are the products of oxidation of the following compounds? Another problem…..Page 213 Problem 12 17 Side Chain Bromination Reaction of an alkylbenzene with N-bromo-succinimide (NBS) and benzoyl peroxide (radical initiator) introduces Br into the side chain. 18 Reduction of Aromatic Compounds 19 Reactions of Benzene: Electrophilic Substitution Vs Electrophilic Addition 20 Electrophilic Aromatic Substitution 21 22 Halogenation 23 Nitration and Sulfonation 24 Friedel-Crafts Alkylation 25 Limitations of Friedel Crafts Reactions 26 Page 212 Problem 11 27 Friedel-Crafts Acylation 28 Di- and Polysubstitution Existing groups on a benzene ring influence further substitution in both orientation and rate Orientation certain substituents direct new substitution preferentially toward ortho-para positions, others direct preferentially toward meta positions Rate certain substituents are activating toward further substitution, others are deactivating toward further substitution 29 Ortho-para Directors -OCH3 is ortho-para directing 30 Meta Directors 31 Types of Substituents 32 Page 218 Problem 18 33 Page 221 Problem 19 34 Trisubstituted Benzenes 35 36 37 Where would the electrophile attack the ring preferentially? 38 Predict the major product of each of the following reactions. 39 Synthesis of p-Bromobenzoic acid 40 The order of steps is important. 41