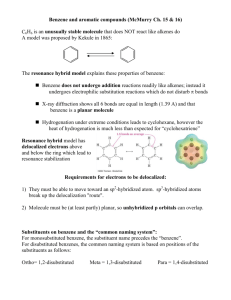
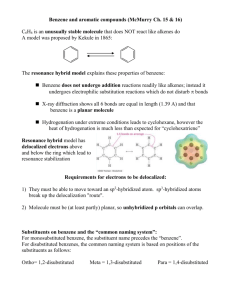
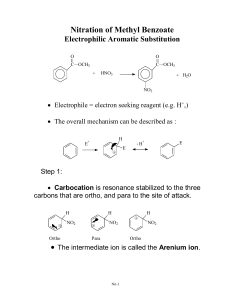
Resonance stabilization
advertisement

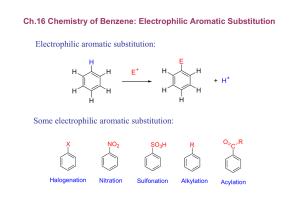
Chapter 17 Aromatic Substitution Reactions OrgChemChap17 1 17.1 Mechanism for Electricphilic Aromatic Substitution Arenium ion resonance stabilization OrgChemChap17 2 Example 1. Example 2. OrgChemChap17 3 Example 2. Mechanism of the nitration of benzene OrgChemChap17 4 Addition reaction vs. Electrophilic aromatic substitution OrgChemChap17 5 < Stability < E H E Ga < Gs OrgChemChap17 < Bezene is very stable so it is very diificult to break the resonance stabilization 6 Is the addition reaction possible for a benzene ? Very difficult because of the stability of the product E resonance stabilization OrgChemChap17 7 17.2 Effect of Substituent 17 times faster than the substitution of benzene Why ? Resonance stabilization OrgChemChap17 8 Ortho attack Meta attack Para attack Meta and para attack is favored OrgChem-Chap17 9 CH3 is an ortho/para directing group Nitration of anisole (methoxy benzene) 10,000 times faster than the substitution of benzene Why ? Resonance stabilization OrgChemChap17 10 The effect of methoxy group 1.Inductive effect, then as the oxygen is electronegative Methoxy is deactivating group not true 2. Resonance effect explanation is possible This is what scientists are doing, you also should have this attitude, then find reasons. Otherwise no result at all. Therefore, any group that has an unshared pair of electrons is the ortho/para director OrgChemChap17 11 Nitration of nitrobenzene 1. 1017 times slower than the substitution of benzene 2. meta director OrgChemChap17 12 OrgChemChap17 13 Until now, Activating group (elecron donating group): ortho/para director Deactivationg group (elecron withdrawing group): meta dircectot Exception: Halogens, ortho/para derector + deactivating group 1. 17 times slower than the substitution of benzene 2. ortho/para director OrgChemChap17 14 F is highly electronegative, therefore F inductive withdrawing effect is stronger than the resonance effect Cl, Br, I Cl, Br, and I are not very electronegative, while the resonance effect is not strong enough as the methoxy Because the overlapping netween 2p AO of carbon and 3p(Cl), 4p(Br), 5p(I) AOs are not good. (2p AO for oxygen) Still halogens are ortho/para director because there is the resonance effect although it is much weaker. Nose ring theory ! OrgChemChap17 Accurate experiment results are most important ! 15 @ Two ortho positions and one para position, therefore statistically the ratio or ortho to para products should be 2 to 1, Which is generally true! (nitration of toluene) OrgChemChap17 16 See P 680 OrgChemChap17 17 17.3 Effect of Multiple Substituent Methyl group controls the regiochemistry, because methyl group is a strong activating group Rule: Groups that are closer to the top of Table 17.1 controls the regiochemistry! OrgChemChap17 18 17.4 Nitration OrgChemChap17 19 Preparation of NO2+ OrgChemChap17 20 A problem occurs with amino substitution N with unpaired electrons looks like a activating group and o/p director. But under acidic condition it can be protonated, then deactivating group and m director. Although the amine (strong activating group) conc. is very low, 18% is para product! OrgChemChap17 21 Amide group: much less basis, still activator and o/p director Example, OrgChemChap17 22 17.5 Halogenation Mechanism Same as the nitration Cl Resonance stabiliztion, Activating group faciliate the reaction OrgChem-Chap17 + AlCl3 + HCl 23 OrgChemChap17 24 17.6 Sulfonation Fuming sulfuric acid OrgChemChap17 25 Mechanism OrgChemChap17 26 17.7 Friedel-Craft Alkylation OrgChemChap17 27 Mechanism of the Friedel-Craft Alkylation OrgChemChap17 28 Drawbacks 1. The alkyl groups that is added to the ring is an activated group: a large amount of products w/ two or more alkyl groups 2. Aromatic compound w/ strongly deactivating groups cannot be alkylated. 3. Rearrangement CH3CH2CH2CH2Cl + AlCl 3 CH3CH2CH2CH2 AlCl 4 Because OrgChemChap17 CH3CH2CHCH3 29 Other ways to generate carbocations Strong acid, TsOH, can eliminate water, then CH3-ph-CH2+ can be generated Other examples Lewis acid is used OrgChemChap17 30 Synthetic detergents OrgChemChap17 31 BHT and BHA are anti oxidant added to food prepared by Friedel-Crafts alkylation reactions OrgChemChap17 32 17.8 Friedel-Craft Acylation Generation of acyl cation OrgChemChap17 33 Drawback: like the alkylation, this reaction does not work with strongly deactivated substrates (m directors) Examples OrgChemChap17 34 Examples OrgChemChap17 35 17.9 Electrophilic Substitution of Polycyclic Aromatic Compounds Why the 1 position is preferred? OrgChemChap17 36 Containing stable benzene ring Containing stable benzene ring OrgChemChap17 37 17.10 Nucleophilic Aromatic Substitution; Diazonium ion OrgChemChap17 38 Examples OrgChemChap17 39 17.11 Nucleophilic Aromatic Substitution; Addition-Elimination OrgChemChap17 40 Mechanism OrgChemChap17 Not SN2 but Addition-Elimination 41 The order of leaving group ability Examples OrgChemChap17 42 17.12 Nucleophilic Aromati Substitution; Elimination-Addition When there is no electron withdrawing group at o/p position, then elimination-addition occurs with very strong base (amide anion) or with weak base at high temperature OrgChemChap17 43 Mechanism OrgChemChap17 44 Benzyne The existence of benzyne OrgChemChap17 45 17.13 Some Additional Useful Reactions Reduction of nitro group to amine using hydrogen and a catalyst or by using acid and a metal (Fe, Sn, or SnCl2) O H3CH2COC NH2 Cl Application OrgChemChap17 46 Reduction of carbonyl group (aldehyde or ketone) to a methylene group 1. Clemmenson reduction 2. Wolff-Kishner reduction 3. Catalytic hydrogenation OrgChemChap17 47 H2/Pt reduction vs Wolff-Kishner and Clemmenson reduction -H2/Pt works for the carbonyl attached to the aromatic ring -Wolff-Kishner and Clemmenson reduction do not have this restriction Oxidation of alkyl groups bonded to the aromatic ring OrgChemChap17 If the carbon bonded to the ring is not tertiary 48 17.14 Synthesis of Aromatic Compound OrgChemChap17 49 Preparation of m-chlorobenzene and p-chlorobenzene Preparation of o-bromophenol HO HO HO + Br2 Br + Mixuture Br OrgChemChap17 50 Preparation of m-bromochlorobenzene Problem: both chloro and bromo groups are o/p directors Solution: use NO2, a m director Preparation of m-bromotoluene Problem: methyl group is an o/p director Solution: use NO2, the m director OrgChemChap17 51 Preparation of m-butylbenzenesulfonic acid Benzene sulfonic acid cannot be alkylated because the FriedelCraft alkyl- or acylation does not work with deactivating group OrgChemChap17 52 Preparation of OrgChemChap17 bezene53