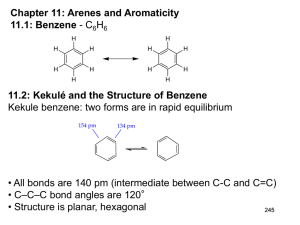
Substituent Effects in Electrophilic Aromatic Substitution

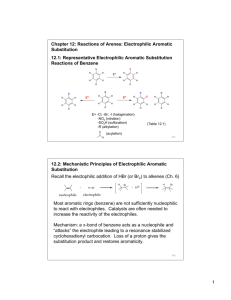
Chapter 12: Reactions of Arenes: Electrophilic Aromatic
Substitution
12.1: Representative Electrophilic Aromatic Substitution
Reactions of Benzene
E= -Cl, -Br, -I (halogenation)
-NO
2
(nitration)
-SO
3
H (sulfonation)
-R (alkylation)
(acylation)
(Table 12.1)
12.2: Mechanistic Principles of Electrophilic Aromatic
Substitution
Recall the electophilic addition of HBr (or Br
2
) to alkenes (Ch. 6)
H H Br
+ Br nucleophile
+
H Br electrophile
Most aromatic rings (benzene) are not sufficiently nucleophilic to react with electrophiles. Catalysts are often needed to increase the reactivity of the electrophiles.
Mechanism: a
-bond of benzene acts as a nucleophile and
“attacks” the electrophile leading to a resonance stabilized cyclohexadienyl carbocation. Loss of a proton gives the substitution product and restores aromaticity.
H
H
H
H
H
H
+ E-X
H
HX +
H
H
E
H
H
Electrophilic substitution: product regains aromatic stabilization
H
H
H
E
H
H
X
H
Resonance stabilized cyclohexadienyl cation intermediate
Aromaticity is worth ~ 130-150 KJ/mol
H
H
H
E
H
X
H
H
H
E
H
+
H
X
H
H
Electrophilic addition: products lose aromatic stabilization
12.3: Nitration of Benzene
HNO
3
, H
2
SO
4
H
2
O
HO N
O
O
Nitric acid pK a
~ -1.3
+ H
2
SO
4
Sulfuric acid pK a
~ -2.0
O
H
2
O N
O
O
N
O
O
N
O
Nitronium
Ion
+ H
2
O
12.4: Sulfonation of Benzene
SO
3
, H
2
SO
4
H
2
O
O S
O
O
+ H
2
SO
4
O
S
O
OH sulfur analogue of a carboxylic acids
Benzene sulfonic acid
HO S
O
O
+ HSO
4
-
12.5: Halogenation of Benzene
Cl
2
, FeCl
3
Cl
For X= Cl or Br
X X
FeX
3
I
2
+ Cu
2+
Br
2
, FeBr
3
I
2
, CuCl
2
Br
I
+
X
-
X FeX
2 I
+
+ 2 Cu
+
12.6: Friedel-Crafts Alkylation of Benzene
Cl
AlCl
3
+ + HCl
Cl alkyl halide
(electroiphile)
AlCl
3 strong
Lewis acid carbocation
+ AlCl
4
Since the Friedel-Crafts alkylation goes through a carbocation intermediate, skeletal rearrangements of the alkyl halide are common
AlCl
3
+ Cl
+
(not observed)
+
Cl
AlCl
3
(65 %)
+
(35 %)
alkyl halide: halide = F, Cl, Br, I must be an alkyl halide; vinyl and aryl halides do not react the aromatic substrate: can not have strong electron withdrawing substituents, nor an amino group
Y
Y ≠ NO
2
, C
N, -SO
3
H
O (R= ketone, aldehyde, carboxylic acids,
R ester)
-NH
2
, NHR, NR
2,
-N + R
3
,
F-C alkylation is often difficult to stop after one alkylation reaction
+ (H
3
C)
3
C-Cl
AlCl
3
(H
3
C)
3
C-Cl
AlCl
3 initial product is more reactive than benzene major product
12.7: Friedel-Crafts Acylation of Benzene
O
O
AlCl
3
+
C
Cl R
R
R
O
C
Cl
+ AlCl
3
O
C
R
O
C
R
AlCl
4
O
C
R acyl group
The acylated product is less reactive than benzene toward electrophilic aromatic substitution. F-C acylation can be stopped after one acyl group is added
12.8: Synthesis of Alkylbenzenes by Acylation-Reduction
Ketones and aldehydes can be reduced to the alkanes with:
Zn(Hg), HCl (Clemmensen Reduction)
H
2
NNH
2
, KOH (Wolff-Kishner Reduction)
O
Zn(Hg), HCl
-or-
H
2
NNH
2
, KOH
Aryl Ketone
AlCl
3
Cl
+ +
O
AlCl
3
Cl
O
Zn(Hg), HCl
-or-
H
2
NNH
2
, KOH
Rearrangements and multiple alkylations are not observed for the F-C acylation
+
12.9: Rate and Regioselectivity in Electrophilic Aromatic
Substitution The nature of a substituent already present on the benzene ring affects the rate and regioselectivity (relative position) of electrophilic aromatic substitution.
A substituent (-X) is said to be activating if the rate of electrophilic aromatic substitution of the substituted benzene (C
6
H
5
X) is faster than benzene.
A substituent (-X) is said to be deactivating if the rate of electrophilic aromatic substitution of the substituted benzene
(C
6
H
5
X) is slower than benzene.
Relative rate of nitration:
CF
3
CH
3
(trifluoromethyl)benzene benzene toluene
2.5 x 10 -5 1 20-25 deactivating activating
CH
3 toluene
H
2
SO
4
, HNO
3 o
CH
3
NO
2
+
-nitrotoluene
(63%)
CH
3
CH
3 m -nitrotoluene
(3%)
NO
2
+
NO
2 p -nitrotoluene
(34%)
CF
3
CF
3
CF
3
CF
3
H
2
(trifluoromethyl)benzene
SO
4
, HNO
3
NO
2
+ +
NO
2 o -nitro-(trifluoromethyl) benzene
(6%) m -nitro-(trifluoromethyl) benzene
(91%)
NO
2 p -nitro-(trifluoromethyl) benzene
(3%)
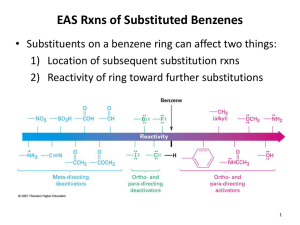
A substituent (-X) is said to be an ortho-para director if it directs an incoming electrophile to positions ortho and/or para to itself.
A substituent (-X) is said to be an meta director if it directs an incoming electrophile to position meta to itself.
Substituents are characterized as either electron-donating or electron-withdrawing and alter the electron density of the aromatic ring through:
1. Inductive effects: ability of a substituent to donate or withdraw electron density through
-bonds due to electronegativity differences and bond polarities of a functional group
+
X
+
C
-
N
O
-
C
+
R
O
-
N
O
-
CH
3
X= F, Cl,
Br, I
Electron-withdrawing groups Electron-donating group
2. Resonance effects: ability of a substituent to donate or withdraw electrons through non-bonding pairs of electrons or overlap
-bonds (conjugation).
C
N
O
C
R
O
N
O
X OCH
3
Electron-withdrawing groups Electron-donating groups
The rate (activating or deactivating) and regiochemistry
(ortho-para vs meta directing) can be understood by examining the influence of the substituent on the stability of the cyclohexadienyl cation intermediate.
12.10: Rate and Regioselectivity in the Nitration of Toluene :
Regioselectivity: The carbocation intermediate from o - or p -addition can be stabilized by the substituent through inductive effects and hyperconjugation.
ortho
63%
CH
3 meta
3% para
34%
Activating groups increase the rate of electrophilic aromatic substitution at all positions of the ring.
Partial rate factors - relative rate of electrophilic aromatic substitution compared to benzene
CH
3
H
3
C
H
3
C
C
CH
3
42
2.5
42
2.5
4.5
3
4.5
3
58 75
Electron rich aromatic rings are more nucleophlic.
All activating group donate electrons through inductive effects and/or resonance. Electron-donating groups stabilize the carbocation intermediate of electrophilic aromatic substitution.
12.11: Rate and Regioselectivity in the Nitration of
(Trifluoromethyl)benzene - Regioselectivity: The carbocation intermediate from o - or p -addition is destabilized by the electron-withdrawing substituent. This directs addition to the m -position. ortho
6%
CF
3 meta
91% para
3%
Dactivating groups decrease the rate of electrophilic aromatic substitution at all positions of the ring.
Partial rate factors - relative rate of electrophilic aromatic substitution compared to benzene
CF
3
4.5 x 10
-6
6.7 x 10
-5
4.5 x 10
-6
4.5 x 10
-6
6.7 x 10
-5
Electron deficient aromatic rings are less nucleophlic.
All deactivating group withdraw electrons through inductive effects and/or resonance. Electron-withdrawing groups destabilize the carbocation intermediate of electrophilic aromatic substitution.
12.12: Substituent Effects in Electrophilic Aromatic
Substitution: Activating Substituents
All activating substituents increase the rate of electrophilic aromatic substitution and are ortho-para directors.
Nitration of phenol: the -OH is a very strong activating group ortho
50%
OH meta
0% para
50%
Substituents that have an O or N atom directly attached to the aromatic ring are strong activators. Phenol, anisole, and anilines are very strong activators and do not require strong Lewis Acid catalysts to undergo electrophilic aromatic substutution.
O
-alkyl, -vinyl, -aryl -OH, -OCH
O C R
O
H
N C R
3
, -NH
2 activators strong activators very strong activators
12.13: Substituent Effects in Electrophilic Aromatic
Substitution: Strongly Deactivating Substituents
Strong deactivators are meta directors
O
C H
O
C R
O
C OH
O
C OR C N
O
S O
O
N
O
O
CF
3 strong deactivators very strong deactivators
12.14: Substituent Effects in Electrophilic Aromatic
Substitution: Halogens - Halogens are deactivating because they are strong electron withdrawing groups (inductive effect); however, they have non-bonding pairs of electrons and can also donate electrons (resonance effect ), and are ortho-para directors.
ortho
30%
Cl meta
1% para
69%
Table 12.2, p. 491
-NO
2
-SO
3
H -CO
2
H
O
C H
-Br -F
-NR
3
C N
O
C R strong deactivators
(meta directors)
-CO
2
R -I -Cl -H deactivators
(ortho/para directors) alkyl
O
O C R
-OR -NH
2
O
H
N C R
-OH strong activators
(ortho/para directors)
12.15: Multiple Substituent Effects - The individual directing effect of each substituent must be considered in order to determine the overall directing effect of a disubstituted benzene toward further electrophilic substitution.
1. When the individual directing effects of the two groups reinforce, further electrophilic substitution is directed to the common position.
-CH
3
directs here
CH
3
-CH
3
directs here
CH
3
-NO
2
directs here -NO
2
directs here
Br
2
, FeBr
3
Br
NO
2
NO
2
2. When the individual directing effects of two groups oppose, the stronger activating group has the dominant influence; however, mixtures of products are often produced.
-OH directs here OH -OH directs here
OH
Br
2
, FeBr
3
Br
-CH
3
directs here -CH
3
directs here
CH
3
CH
3
3. Further substitution between two existing substituents rarely occurs. Start with an ortho-disubstituted benzene to synthesize 1,2,3-trisubstituted benzenes
-CH
3
directs here
-Cl directs here
CH
3
-CH
3
directs here
-Cl directs here
Br
2
, FeBr
3
Br
CH
3
+
CH
3
+
CH
3
Br
Cl
Cl
Cl Cl
Br not observed
-CH
3
directs here -Cl directs here
-Br directs here
-CH
3
directs here
CH
3
-Cl directs here
Br
Br
-Br directs here
-Br directs here
-CHO directs here
-CH
3
directs here
CHO
Br
-Br directs here
Cl
2
, FeCl
3
-Br directs here
Cl
-Cl directs here
CHO
Br
+
CHO
Br
-Br directs here
-CHO directs here
Cl Cl
12.16: Regioselective Synthesis of Disubstituted Aromatic
Compounds
Consider the directing effects of the substituents to determine the order of their introduction to ensure the correct orientation
Friedel-Crafts reactions (alkylation, acylation) cannot be carried out on strongly deactivated aromatics
Sometimes electrophilic aromatic substitution must be combined with a functional group transformation
Br
CO
2
H
NO
2
o,p-director deactivating
Cl
NO
2 m-director deactivating o,p-director activating
Summary of electrophilic aromatic substitution of benzene
Zanger, M.; Gennaro, A. R.; McKee, J. R. J. Chem. Ed. 1993 , 70 (12) , 985-987
SO
3
,
H
2
SO
4
SO
3
H
(m-)
X
X
2
, catalyst
HNO
3
,
H
2
SO
4
NO
2
RCH
2
X,
AlCl
3
CH
2
R
[H]
O
RCOCl,
AlCl
3
R
(o-, p-) (m-) (o-, p-)
NBS, h
Br R
[O]
CO
2
H
(m-)
[O]
(o-, p-) (m-)
12.17: Substitution in Naphthalene (please read)
12.18: Substitution in Heterocyclic Aromatic Compounds
(please read) 301